Throat Lozenges Monograph
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
Date
2018-12-07
Foreword
This monograph is intended to replace the existing Throat lozenges Monograph of January 13, 1995. This monograph describes the requirements necessary to receive market authorization [i.e. a Drug Identification Number (DIN) or a Natural Product Number (NPN)], for throat lozenges products. The monograph identifies the permitted medicinal and non-medicinal ingredients, concentrations, indications, directions and conditions of use for these products to be market authorized without the submission to Health Canada of additional evidence. It also may contain the test methods recommended to be used to comply with the requirements of this monograph. Products which do not meet the criteria outlined in this document can apply for market authorization outside of the monograph stream.
Applicants are reminded that throat lozenges products, like other non-prescription drugs or natural health products, are subject to the Food and Drug Regulations or the Natural Health Products Regulations administered by the Natural and Non-prescription Health Products Directorate (NNHPD). This includes requirements related to labelling, manufacturing and product specifications. Additional information on labels, outside of those specified in the monograph, such as additional directions for use and/or non-therapeutic claims are acceptable as long as they meet the Guidelines for the Nonprescription and Cosmetic Industry Regarding Non-therapeutic Advertising and Labelling Claims, the Guidelines for Consumer Advertising of Health products for Nonprescription drugs, Natural Health Products, Vaccines and Medical Devices, and are not false, misleading or counterintuitive to the use of the product.
The development of this monograph is the result of a thorough review of existing regulations, guidance documents, policies and current practices within Health Canada and other leading regulatory agencies.
Notes
The solidus (/) indicates that the terms and/or the statements are synonymous. Either term or statement may be selected by the applicant. Text in parentheses is additional optional information which can be included on the Product Licence Application form and label at the applicant’s discretion.
Medicinal Ingredient(s)
Throat lozenges products are classified as natural health products (NHPs) if they contain only ingredients from Table 1, 2 and 3. Applicants applying for an NPN can access the appropriate forms and guidance at:https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription.html.
Throat lozenges products are classified as non-prescription drugs if they contain at least one ingredient from Table 4 and 5. Applicants applying for a DIN can access the appropriate forms and guidance at:https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents.html
| Proper name(s) | Common name(s) | Source material(s) | Quantity per lozenge |
|---|---|---|---|
| Common name(s) | |||
alpha-Hydroxytoluene |
Benzyl alcohol |
Benzyl alcohol |
100 - 500 mg |
4-Aminobenzoic acid, ethyl ester |
Benzocaine |
Para-aminobenzoic acid |
2 - 15 mg |
2-Hydroxybenzenemethanol |
Salicyl alcohol |
Salicyl alcohol |
50 - 100 mg |
|
|
dl-Menthol |
2 - 20 mg |
|
|
l-Menthol |
2 - 20 mg |
Phenol |
Phenol |
Phenol |
10 - 50 mg |
| Proper name(s) | Common name(s) | Quantity per lozenge | |||
|---|---|---|---|---|---|
| Proper name(s) | Common Name(s) | Part(s) | |||
Gelatin |
|
N/A |
Gelatin |
N/A |
≤100% |
Pectin |
Pectin |
N/A |
Pectin |
N/A |
≤100% |
Ulmus rubra |
|
Ulmus rubra |
N/A |
Stem bark inner |
10-15% |
| Proper name(s) | Common name(s) | Quantity per lozenge | ||
|---|---|---|---|---|
| Proper name(s) | Part(s) | |||
Eucalyptus globulus |
|
Eucalyptus globulus |
Leaf |
0.2-15 mg |
Table 2 Footnotes
|
||||
| Proper name(s) | Common name(s) | Quantity per lozenge | |
|---|---|---|---|
| Common name(s) | |||
4-n-Butoxy-beta-(1-piperidyl)propiophenone hydrochloride |
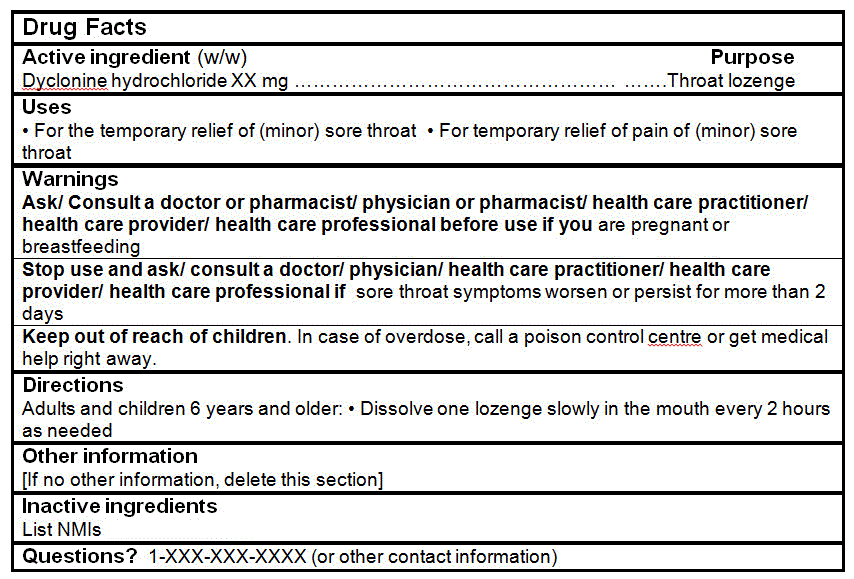
Dyclonine hydrochloride |
Dyclonine hydrochloride |
1-3 mg |
|
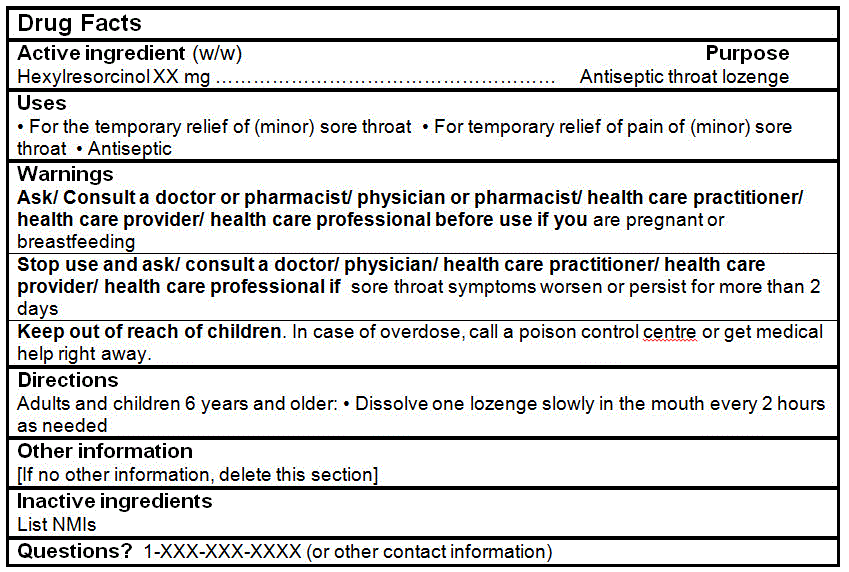
Hexylresorcinol |
Hexylresorcinol |
2-4 mg |
| Proper name(s) | Common name(s) | Quantity per lozenge | |
|---|---|---|---|
| Common name(s) | |||
1-Hexadecylpyridinium chloride |
Cetylpyridinium chloride(antiseptic) |
Cetylpyridinium chloride |
1-2 mg |
|
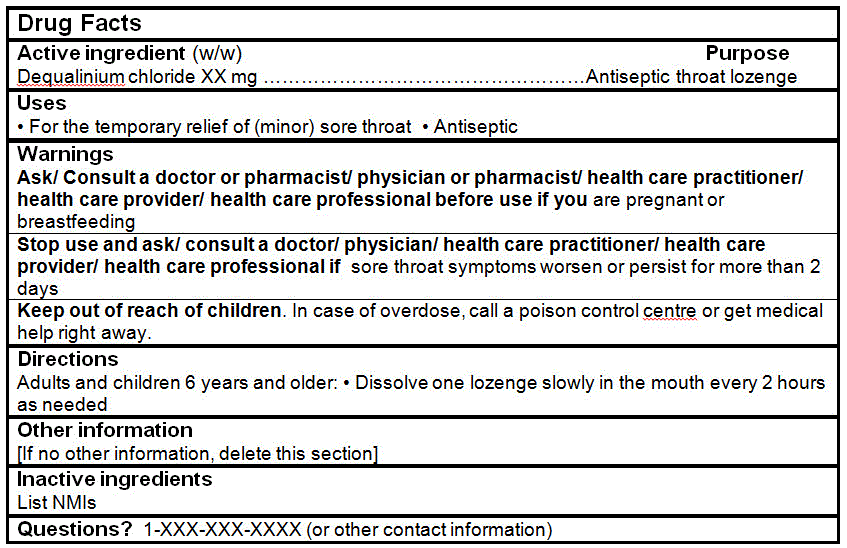
Dequalinium chloride (antiseptic) |
Dequalinium chloride |
0.25 mg |
|
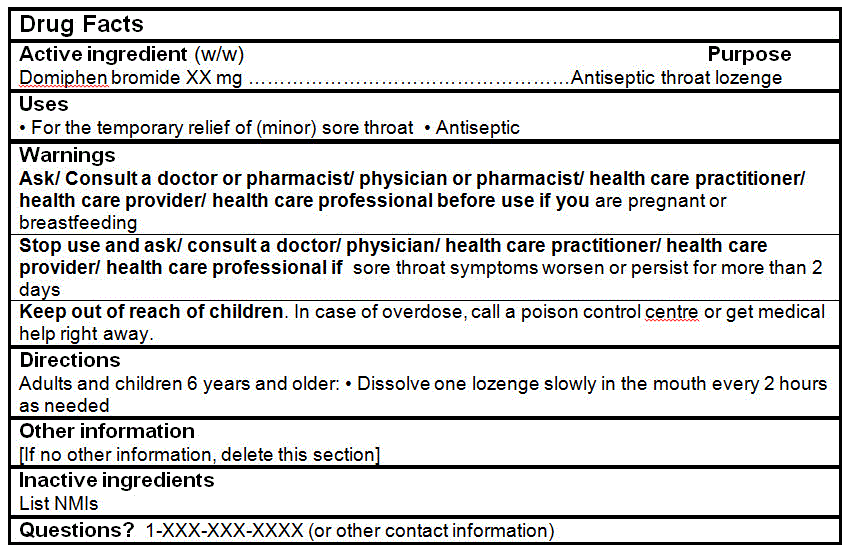
Domiphen bromide(antiseptic) |
Domiphen bromide |
1.5 mg |
|
Hexylresorcinol (analgesic/anesthetic/antiseptic) |
Hexylresorcinol |
2-4 mg |
Route of Administration
Oral
Dosage Form(s)
Lozenges
Use(s) or Purpose(s)
Self-Care Framework Category I Uses or Purposes:
For all products:
- For the temporary relief of (minor) sore throat.
For products containing menthol, phenol, hexylresorcinol, benzocaine, dyclonine hydrochloride, benzyl alcohol, salicyl alcohol:
- For temporary relief of pain of (minor) sore throat.
For products containing slippery elm bark powder, gelatin, or pectin:
- For the protection of irritated area in throat.
For products containing menthol with a concentration of 1 - 20 mg:
- Helps ease/relieve nasal congestion
- Makes nasal passages feel clearer
For products containing menthol with a concentration of 5 - 20 mg:
- For the temporary relief of coughs
For products containing hexylresorcinol, cetylpyridinium chloride, domiphen bromide, phenol, or dequalinium chloride:
- Antiseptic
Dose(s)
Subpopulation(s)
Children 6 to 11 years, Adolescents 12 to 17 years, Adults 18 years and older
Quantity(ies)
See Tables 1, 2, 3, 4 and 5 above.
Permitted combinations:
The concentration of the individual ingredients must not exceed the maximum value permitted as single medicinal ingredients, as listed in Table 1, 2, 3, and 4 above.
- menthol + eucalyptus oil
- menthol + hexylresorcinol
- benzocaine + cetylpyridinium chloride
- benzocaine + menthol
- benzocaine + phenol
- any demulcent+ any anaesthetic/analgesic
- any demulcent + any antiseptic
Direction(s) for use
For products containing menthol, eucalyptus oil, gelatin or pectin:
- Dissolve one lozenge slowly in the mouth as required.
For products containing phenol:
- Dissolve one lozenge slowly in the mouth every 2 hours as needed to a maximum daily dose of 300 mg [as expressed in number of lozenges]
For products containing hexylresorcinol, benzyl alcohol, dyclonine hydrochloride, cetylpyridinium chloride, domiphen bromide, dequalinium or salicyl alcohol:
- Dissolve one lozenge slowly in the mouth every 2 hours as needed
For products containing slippery elm bark powder:
- Dissolve one lozenge slowly in the mouth every 1 - 2 hours up to a maximum of 6 g per day [as expressed in number of lozenges]
For products containing benzocaine:
- Dissolve one lozenge slowly in the mouth. Do not bite, chew or swallow whole. May be repeated every two hours as needed up to a maximum of 3 mg/kg body weight per day [as expressed in number of lozenges] (USP DI 2006; US FDA 1991).
- Avoid eating or drinking while numbness persists.
- Children under 12 years of age should be supervised by an adult in the use of this product.
Duration(s) of Use
For products containing benzocaine:
- For occasional use only
Risk Information
Caution(s) and warning(s)
For all products:
- Keep out of reach of children. In case of overdose, call a poison control centre or get medical help right away.
For products including claim for the temporary relief of (minor) sore throat and/or temporary relief of pain of (minor) sore throat:
- Stop use and ask/ consult a doctor/ physician/ health care practitioner/ health care provider/ health care professional if sore throat symptoms worsen or persist for more than 2 days.
For products including claim for the temporary relief of coughs:
- Stop use and ask/ consult a doctor/ physician/ health care practitioner/ health care provider/ health care professional if cough worsens and persists for more than 7 days, or is accompanied by a fever.
For products containing ingredients in Tables 4 or 5:
- Ask/ Consult a doctor or pharmacist/ physician or pharmacist/ health care practitioner/ health care provider/ health care professional before use if you are pregnant or breastfeeding.
For products containing benzocaine:
- Stop use and ask/ consult a doctor/ physician/ health care practitioner/ health care provider/ health care professional if sore throat is severe, persists for more than two days, or is accompanied by or followed by other symptoms such as fever, headache, rash, swelling, nausea, or vomiting (Pray 2006; USP DI 2006; US FDA 1991).
Contraindication(s)
For products containing benzocaine:
- Do not use if allergic to benzocaine.
Known adverse reaction(s)
For products containing benzocaine:
- Allergy alert Hypersensitivity/allergy is known to occur. In such a case, discontinue use / Allergy alert Stop use if hypersensitivity/allergy occurs (HC 2011a;b US FDA 1991).
Non-medicinal ingredients
Ingredients must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in that database, the Food and Drug Regulations (FDR), and the current Cosmetic Ingredient Hotlist, when relevant.
Storage conditions
No statement required.
Specifications
This monograph describes those requirements that are specific to this class of non-prescription drugs and to natural health products (NHPs). Any change to the manufacturing process that impacts the safety and efficacy of the ingredients, such as the use of novel technology (e.g. nanotechnology), requires supporting data and will be reviewed outside the monograph.
For products containing Table 1, 2 and 3 NHP medicinal ingredients only:
The finished product specifications must be established in accordance with the requirements described in the NNHPD Quality of Natural Health Products Guide. The medicinal ingredient must comply with the requirements outlined in the NHPID.
For products containing Table 4 and 5 non-prescription drug medicinal ingredients:
Requirements described in the Regulations to the Food and Drugs Act must be met.
DRUG FACTS TABLES (Format Optional for Self-Care Category I)

References
- Compendium of therapeutics for minor ailments (CTMA). Power B et al., editors. Canadian Pharmacists Association ; 2016
- Compendium of products for minor ailments (CPMA). Power B, et al., editors. Canadian Pharmacists Association ; 2016
- Federal Register vol 47, No 101 May 25, 1982. pp. 22779-22905, Over the Counter Oral Healthcare and Discomfort Drugs, Establishment of a Monograph.
- Federal Register vol 53, No 17, January 27, 1988. pp. 2457-2461, Oral Healthcare Products for Over the Counter Human Use, Proposed Rules.
- Federal Register vol 52, No 155, August 12, 1987. pp. 30045-30057, Final Monograph for Over the Counter Antitussive Drug Products.
- Federal Register vol 55, No 192, October 3, 1990. pp. 40381-40383, as above.
- Federal Register vol 41, No 176, September 9, 1976. pp. 38405-38424, Cough and Cold, Allergy, Bronchodilator and Antihistamine Products for Over the Counter Use.
- HC 2011a: Health Canada 2011. Health risks associated with the use of topical benzocaine products. Internal document. Available on Request.
- HC 2011b: Health Canada advises Canadians of health risks involved with using benzocaine products. About Health Canada. [Updated: April 19, 2011; Accessed 2013 January 15].
- Krinsky DL, Ferreri SP, Hemstreet B, Hume AL, Newton GD, Rollins CJ, Tietze KJ, Handbook of Nonprescription Drugs: An interactive approach for Self-Care, 19th edition. Washington (DC): American Pharmaceutical Association; 2017
- Principles and Practice of Disinfection, Preservation and Sterilisation, edited by AD Russell, WB Hugo and GAJ Ayliffe, 1982. Blackwell Scientific Publications.
- Remington: The Science and Practice of Pharmacy; 22nd edition. Philadelphia, PA; Pharmaceutical Press 2012
- Third Report of the Expert Advisory Committee, Nonprescription Cough Cold Remedies, Ministry of National Health and Welfare, 1989. pp. 4-9.
- US FDA 2011a: FDA continues to receive reports of a rare, but serious and potentially fatal adverse effect with the use of benzocaine sprays for medical procedures 04-07-2011 [Internet]. [Accessed 2011 June 6].
- US FDA 2011b: FDA Drug Safety Communication: Reports of a rare, but serious and potentially fatal adverse effect with the use of over-the-counter (OTC) benzocaine gels and liquids applied to the gums or mouth 04-07-2011. [Internet]. [Accessed 2011 June 6].
- US FDA 2011c: Benzocaine Topical Products: Sprays, Gels and Liquids - Risk of Methemoglobinemia. [Posted 04/07/2011] U.S. Food and Drug Administration MedWatch The FDA Safety Information and Adverse Event Reporting Program. [Internet]. [Accessed 2011 December 21].
- US FDA 1991: US Department of Health and Human Services, Food and Drug Administration. 21 CFR Parts 356 and 369. [Docket No. 81N-0033]. Oral Health Care Drug Products for Over-the-Counter Human Use; Amendment to Tentative Final Monograph to Include OTC Relief of Oral Discomfort Drug Products. ACTION: Notice of proposed rulemaking. [Internet]. Federal Register, Volume 56, Number 185, September 24, 1991. [Accessed 2011 December 14].
- USP DI 2006: Drug Information for the Health Care Professional. 26th edition, Volume 1. Greenwood Village (CO): Thomson Micromedex; 2006.