Diaper Rash Products
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
Date
2024-02-23
Foreword
This monograph is intended to replace the existing Diaper Rash Products Monograph of December 7, 2018. This monograph describes the requirements necessary to receive market authorization [i.e. a Drug Identification Number (DIN) or a Natural Product Number (NPN)], for diaper rash products. The monograph identifies the permitted medicinal and non-medicinal ingredients, concentrations, indications, directions and conditions of use for these products to be market authorized without the submission to Health Canada of additional evidence. Products which do not meet the criteria outlined in this document can apply for market authorization outside of the monograph stream.
Applicants are reminded that diaper rash products, like other non-prescription drugs or natural health products, are subject to the Food and Drug Regulations or the Natural Health Products Regulations administered by the Natural and Non-prescription Health Products Directorate (NNHPD). This includes requirements related to labelling, manufacturing and product specifications. Additional information on labels, outside of those specified in the monograph, such as additional directions for use and/or non-therapeutic claims are acceptable as long as they meet the Guidelines for the Nonprescription and Cosmetic Industry Regarding Non-therapeutic Advertising and Labelling Claims, the Guidelines for Consumer Advertising of Health products for Nonprescription drugs, Natural Health Products, Vaccines and Medical Devices, and are not false, misleading or counterintuitive to the use of the product.
Notes
- Text in parentheses is additional optional information which can be included on the Product Licence Application form and label at the applicant’s discretion.
- The solidus (/) indicates that the terms and/or the statements are synonymous. Either term or statement may be selected by the applicant
Medicinal Ingredient(s)
Diaper rash products are classified as natural health products (NHPs) if they contain ingredients from Tables 1 and 2. Applicants applying for an NPN can access the appropriate forms and guidance at: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription.html.
Diaper rash products are classified as non-prescription drugs (NPDs) if they contain at least one ingredient from Table 3 at a quantity listed in the table. Applicants applying for a DIN can access the appropriate forms and guidance at: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents.html.
| Proper name(s) 1 | Common name(s) 1 | Source information 1 | Quantity 2 | |||
|---|---|---|---|---|---|---|
| Proper name(s) | Common name(s) | Part(s) | Preparation(s) | |||
| (2,5-Dioxo-4-imidazolidinyl)urea |
|
N/A | Allantoin 3 | N/A | N/A | 0.5 - 2% |
| Iron oxide (Fe2O3), mixture with zinc oxide | Calamine | N/A | Calamine 3 | N/A | N/A | 1 - 25% |
| Kaolin |
|
N/A | Kaolin 3 | N/A | N/A | 4 - 20% |
| Zea mays |
|
Zea mays 3 | N/A | Seed | Dry | 10 - 98% |
| Zinc oxide |
|
N/A | Zinc oxide 3 | N/A | N/A |
1 - 25% (Zinc oxide) 25 - 40% (Zinc oxide ointment) |
1
At least one of the following references was consulted per proper name,
common
name, and source material: RSC 2024; USP-NF 2023; Nikitakis and Lange 2016;
Sweetman 2017.
2 At least one of the following references was consulted for the dosage: US FDA
2021; Krinsky 2017; Sweetman 2017; Leung and Foster 2010.
3 Ingredient must be pharmacopoeial grade.
| Proper name(s) 1 | Common name(s) 1 | Source material(s) 1 | Quantity 2 | ||
|---|---|---|---|---|---|
| Proper name(s) | Common name(s) | Part(s) | |||
| Cod liver oil |
|
N/A |
|
Liver | 5 - 14% |
|
|
Ovis aries | N/A | Wool | 15.5% |
1
At least one of the following references was consulted per proper
name,
common
name, and source material: RSC 2024; Sweetman 2017; Nikitakis and Lange
2016;
USP-NF 2023
2
The following reference was consulted for the dosage: US FDA
2021.
3
Cod liver oil and Lanolin are not permitted as single medicinal
ingredients
as
these ingredients are not sufficient on their own to support the efficacy of
the
product.
4
The quantities of vitamin A and vitamin D provided by the cod liver oil in
the
product must not exceed 3000 µg RAE vitamin A (10 000 IU) and 10 µg vitamin
D/cholecalciferol (400 IU) per day (US FDA 2021).
| Proper name(s) | Common name(s) | Quantity |
|---|---|---|
|
Dimethicone | 1 - 30% |
| Mineral oil |
|
50 - 100% |
| Petrolatum |
|
30 - 100% |
| White petrolatum |
|
30 - 100% |
1 See Permitted combinations.
Route(s) of administration
Topical
Dosage form(s)
Acceptable dosage forms for NHPs:
- Cream; Gel; Lotion; Oil; Ointment; Paste; Powder; Salve; Solution; Topical liquid; Wipe, medicated
Acceptable dosage forms for NPDs:
- Cream; Gel; Lotion; Oil; Ointment; Paste; Solution; Wipe
Use(s) Or Purpose(s)
Self-Care Framework Category I Uses or Purposes:
For products with an ingredient from Table 1 or 3:
- Helps prevent diaper rash (US FDA 2021).
- Protects chafed skin/minor skin irritation due to diaper rash (US FDA 2021).
- Helps protect from wetness that causes diaper rash (US FDA 2021).
- Temporarily helps relieve minor skin irritation due to diaper rash (US FDA 2021).
- Helps treat/ heal diaper rash (US FDA 2021).
Dose(s)
Subpopulation(s)
Infants 0 to 1 years, Children 2 to 11 years, Adolescents 12 to 17 years, Adults 18 years and older
Quantity:
See Tables 1, 2, and 3 above.
Permitted combinations:
- Any combination of the ingredients listed in Tables 1, 2 and/or 3 is permitted provided each ingredient in the combination is within the specified quantity (US FDA 2021).
Directions for use:
For all products:
- Apply liberally to a clean and dry diaper area as needed (US FDA 2021).
Duration of use
No statement is required.
Risk information
Caution(s) and warning(s)
For all products:- For external use only (US FDA 2021).
- When using this product avoid contact with eyes. If contact occurs, rinse thoroughly with water (US FDA 2021).
- Stop use and ask a health care practitioner/health care provider/health care professional/doctor/physician if symptoms worsen, last for more than 7 days or re-occur within a few days (US FDA 2021).
- Keep out of reach of children.If swallowed, call a poison control centre or get medical help right away.
- When using this product keep powder away from face to avoid inhalation, which can cause breathing problems (FDA 2003).
Contraindication(s)
For all powder products:
- Do not use on broken skin (FDA 2003).
Known adverse reaction(s):
No statement required.
Non-medicinal ingredients
Ingredients must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in that database, the Food and Drug Regulations, and the current Cosmetic Ingredient Hotlist, when relevant.
Storage Condition(s)
Must be established in accordance with the requirements described in the Health Products Regulations and the Food and Drugs Regulations.
Specifications
This monograph describes those requirements that are specific to this class of non-prescription drugs and to NHPs. Any change to the manufacturing process that impacts the safety and efficacy of the ingredients, such as the use of novel technology (e.g. nanotechnology), requires supporting data and will be reviewed outside the monograph.
For products containing Table 1 and 2 NHP medicinal ingredients only:
The finished product specifications must be established in accordance with the requirements described in the NNHPD Quality of Natural Health Products Guide. The medicinal ingredient must comply with the requirements outlined in the NHPID.
For products containing Table 3 NPD medicinal ingredients:
Requirements described in the Regulations to the Food and Drugs Act must be met.
For products containing Table 1 and 2 NHP medicinal ingredients only:
EXAMPLE OF PRODUCT FACTS:
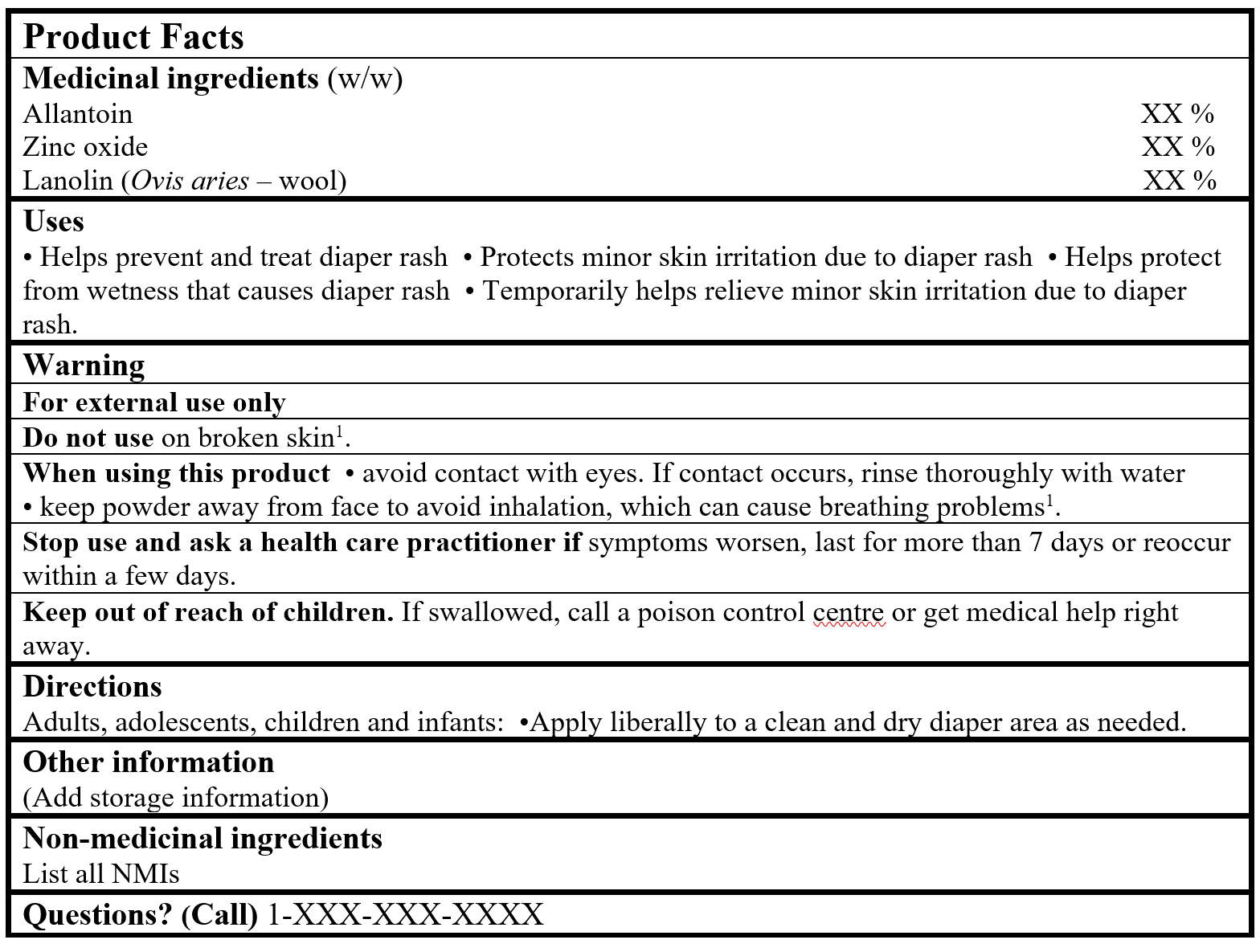
1For powder products
For products containing Table 3 NPD medicinal ingredients:
DRUG FACTS TABLES (Format Optional for Self-Care Category I)




References
- Krinsky DL, Ferreri SP, Hemstreet B, Hume AL, Newton GD, Rollins CJ, Tietze KJ. Handbook of Nonprescription Drugs: An interactive approach for Self-Care, 19th edition. Washington (DC): American Pharmaceutical Association; 2017.
- Leung AY, Foster S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics, 3rd edition. Hoboken (NJ): John Wiley and Sons, Inc.; 2010
- Nikitakis J, Lange B, editors. International Cosmetic Ingredient Dictionary and Handbook. 16th edition. Washington (DC): Cosmetic, Toiletry and Fragrance Association; 2016.
- RSC 2024: Royal Society of Chemistry: The Merck Index Online [Accessed 2024 January 16]. Available from: https://merckindex.rsc.org/
- Sweetman SC, editor. Martindale: The Complete Drug Reference, 39th edition. Grayslake (IL): Pharmaceutical Press; 2017.
- US FDA 2021: U.S. Food and Drug Administration. 21 CFR Part 347. Over-the-Counter (OTC) Monograph M016: Skin Protectant Drug Products for Over-the-Counter Human Use 2021. [Accessed February 2, 2024] Available from: https://www.accessdata.fda.gov/drugsatfda_docs/omuf/OTCMonograph_M016SkinProtectantDrugProductsforOTCHumanUse09242021.pdf.
- USP-NF 2023: United States Pharmacopeia and the National Formulary. Rockville (MD): United States Pharmacopeial Convention, Inc.; 2023.