Creatine Monohydrate
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization. It is not intended to be a comprehensive review of the medicinal ingredient.
Notes
- Text in parentheses is additional optional information which can be included on the PLA and product label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or statements are synonymous. Either term or statement may be selected by the applicant.
Date
March 28, 2024
Proper name(s), Common name(s), Source information
| Proper name(s) | Common name(s) | Source information |
|---|---|---|
| Source ingredient(s) | ||
| N-(Aminoiminomethyl)-N-methylglycine monohydrate | Creatine monohydrate | Creatine monohydrate |
References: Proper name: RSC 2023, US NLM 2023; Common name: RSC 2023, US NLM 2023; Source information: RSC 2023, Weiss and Krommer 1998.
Route of administration
Oral
Dosage form(s)
This monograph excludes foods or food-like dosage forms as indicated in the Compendium of Monographs Guidance Document.
Acceptable dosage forms for oral use are indicated in the dosage form drop-down list of the web-based Product Licence Application form for Compendial applications.
Note
Liquids and solutions are not permitted due to lack of stability of the
finished
product
(Dash and Sawhney 2002).
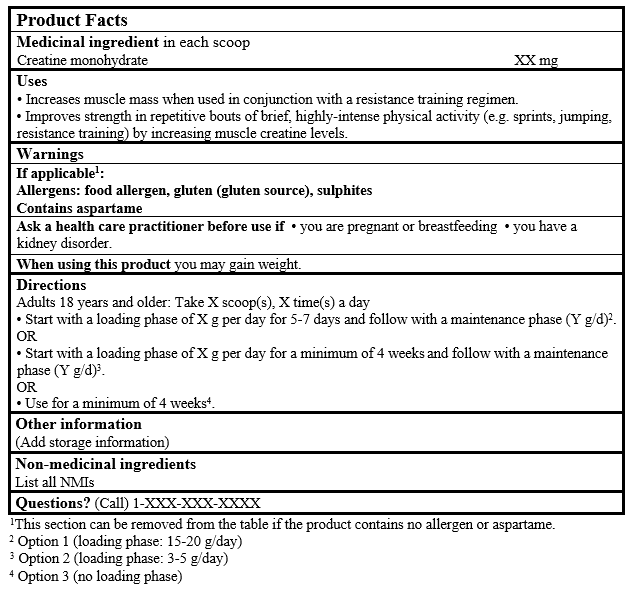
Use(s) or Purpose(s)
- Increases body/(lean)muscle mass/size when used in conjunction with a resistance training regimen (Brose et al. 2003; Bemben et al2001; Volek et al. 1999; Vandenberghe et al. 1997).
- Improves strength/power/performance in repetitive bouts of brief, highly-intense physical activity (e.g. sprints, jumping, resistance training) (by increasing [muscle/intramuscular] [creatine/phosphocreatine/energy] levels) (Okudan and Gökbel 2005; Brose et al. 2003; Preen et al. 2003; Bemben et al. 2001; Volek et al. 1999; Vandenberghe et al. 1997; Hultman et al. 1996).
Dose(s)
Subpopulation(s)
Adults 18 years and older
Quantity(ies)
| Loading Phase | Maintenance Phase | ||||
|---|---|---|---|---|---|
| Min/day | Max/day | Max/single dose | Min/day | Max/day | |
| Option 1 | 15 g | 20 g | 5 g | 2 g | 5 g |
| Option 2 | 3 g | 5 g | N/A | ||
| Min/day | Max/day | |
|---|---|---|
| Option 3 | 3 g | 5 g |
References for Tables 2 and 3: Okudan and Gokbel 2005; Preen et al. 2003; Bemben et al. 2001; Volek et al. 1999; Vandenberghe et al. 1997; Hultman et al. 1996.
Direction(s) for use and duration(s) of use
| Option(s) | Direction(s) for use and duration(s) of use |
|---|---|
| Option 1 - loading phase of 15-20 g/day | Start with a loading phase of X g per day for 5-7 days and follow with a maintenance phase (Y g/day) |
| Option 2 - loading phase of 3-5 g/day | Start with a loading phase of X g per day for a minimum of 4 weeks and follow with a maintenance phase (Y g/day) |
| Option 3 - no loading phase | Use for a minimum of 4 weeks. |
Risk information
Caution(s) and warning(s)
- When using this product you may gain weight (Volek and Rawson 2004; Bemben et al. 2001; Mihic et al. 2000).
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are pregnant or breastfeeding.
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have a kidney disorder (Pline and Smith 2005; Pritchard and Kalra 1998).
Contraindication(s):
No statement required.
Known adverse reaction(s):
No statement required.
Non-medicinal ingredients
Must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in the database.
Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations
Specifications
- The finished product specifications must be established in accordance with the requirements described in the Natural and Non-prescription Health Products Directorate (NNHPD) Quality of Natural Health Products Guide.
- The medicinal ingredient must comply with the requirements outlined in the NHPID.
- The raw material specifications must have limits for the following impurities: not more than 0.1% creatinine; not more than 0.1% dicyandiamide; not more than 0.0005% dihydrotriazine.
EXAMPLE OF PRODUCT FACTS:
Consult the Guidance Document, Labelling of Natural Health Products for more details.
References cited
- Bemben MG, Bemben DA, Loftiss DD, Knehans AW. 2001. Creatine supplementation during resistance training in college football athletes. Medicine & Science in Sports & Exercise 33(10):1667-1673.
- Brose A, Parise G, Tarnopolsky MA. 2003. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. The Journals of Gerontology Series A: Biological Science and Medical Science 58(1):11-19.
- Dash AK, Sawhney A. 2002. A simple LC method with UV detection for the analysis of creatine and creatinine and its application to several creatine formulations. Journal of Pharmaceutical and Biomedical Analysis 29(5):939-945.
- Hultman E, Söderlund K, Timmons JA, Cederblad G, Greenhaff PL. 1996. Muscle creatine loading in men. Journal of Applied Physiology 81(l):232-237.
- Mihic S, MacDonald JR, McKenzie S, Tarnopolsky MA. 2000. Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Medicine & Science in Sports and Exercise 32(2):291-296.
- Okudan N, Gökbel H. 2005. The effects of creatine supplementation on performance during the repeated bouts of supramaximal exercise. Journal of Sports Medicine and Physical Fitness 45(4):507-512.
- Pline KA, Smith CL. 2005. The effect of creatine intake on renal function. The Annals of Pharmacotherapy 39(6):1093-1096.
- Preen D, Dawson B, Goodman C, Beilby J, Ching S. 2003. Creatine supplementation: a comparison of loading and maintenance protocols on creatine uptake by human skeletal muscle. International Journal of Sport Nutrition and Exercise Metabolism 13(1):97-111.
- Pritchard NR, Kalra PA. 1998. Renal dysfunction accompanying oral creatine supplements. The Lancet 351(9111):1252-1253.
- RSC 2023: Royal Society of Chemistry: The Merck Index Online [Accessed 2023 August 14]. Available from: https://merckindex.rsc.org/
- US NLM 2023: United States National Library of Medicine. PubChem. Bethesda (MD): [Creatine monohydrate. RN: 6020-87-7. Accessed 2023 September 28]. Available from: https://pubchem.ncbi.nlm.nih.gov/
- Vandenberghe K, Goris M, Van Hecke P, Van Leemputte M, Vangerven L, Hespel P. 1997. Long-term creatine intake is beneficial to muscle performance during resistance training. Journal of Applied Physiology 83(6):2055-2063.
- Volek JS, Duncan ND, Mazzeti SA, Staron RS, Putukian M, Gomez AL , Pearson DR, Fink WJ, Kraemer WJ. 1999. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Medicine and Science in Sports Exercise 31(8):1147-1156.
- Volek JS, Rawson ES. 2004. Scientific basis and practical aspects of creatine supplementation for athletes. Nutrition 20(7-8):609-614.
- Weiss S, Krommer H. 1998. Process for the preparation of creatine or creatine monohydrate. U.S. Patent 5,719,319.
References reviewed
- Balsom PD, Söderlund K, Ekblom B. 1994. Creatine in humans with special reference to creatine supplementation. Sports Medicine 18(4):268-280.
- Bemben MG, Lamont HS. 2005. Creatine supplementation and exercise performance. Sports Medicine 35(2):107-125.
- Bensky D, Gamble A, Kaptchuk T. 1993. Chinese Herbal Medicine Materia Medica. Vista (CA): Eastland Press.
- Benzi G, Ceci A. 2001. Creatine as nutritional supplementation and medicinal product. Journal of Sports Medicine and Physical Fitness 2001; 41(1):1-10.
- Brudnak MA. 2004. Creatine: are the benefits worth the risk? Toxicology Letters 150(1):123-130.
- Cooke WH, Grandjean PW, Barnes WS. 1995. Effect of oral creatine supplementation on power output and fatigue during bicycle ergometry. Journal of Applied Physiology 78(2):670-673.
- Cottrell GT, Coast JR, Herb RA. 2002. Effect of recovery interval on multiple-bout sprint cycling performance after acute creatine supplementation. Journal of Strength and Conditioning Research 16(1):109-116.
- Cox HE. 1936. Composition of meat extracts and meat cubes. Chemistry and Industry 55:69-71.
- Derave W, Eijnde BO, Hespel P. 2003. Creatine supplementation in health and disease: what is the evidence for long-term efficacy? Molecular and Cellular Biochemistry 244(1-2):49-55.
- Doherty M, Smith PM, Davison RC, Hughes MG. 2002. Caffeine is ergogenic after supplementation of oral creatine monohydrate. Medicine and Science in Sports and Exercise 34(11):1785-1792.
- Edmunds JW, Jayapalan S, DiMarco NM, Saboorian MH, Aukema HM. 2001. Creatine supplementation increases renal disease progression in Han:SPRD-cy rats. American Journal of Kidney Diseases 37(1):73-78.
- Emery JA, Henley RR. 1919. Meat extracts, their composition and identification. Journal of Agricultural Research 17(1):1-17.
- Gotshalk LA, Volek JS, Staron RS, Denegar CR, Hagerman FC, Kraemer WJ. 2002. Creatine supplementation improves muscular performance in older men. Medicine and Science in Sports and Exercise 34(3):537-543.
- Greenwood M, Kreider RB, Melton C, Rasmussen C, Lancaster S, Cantler E, Milnor P, Almada A. 2003. Creatine supplementation during college football training does not increase the incidence of cramping or injury. Molecular and Cellular Biochemistry 244(1-2):83-88.
- Harris RC, Soderlund K, Hultman E. 1992. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clinical Science (London) 83(3):367-374.
- Izquierdo M, Ibanez J, Gonzalez-Badillo J, Gorostiaga EM. 2002. Effects of creatine supplementation on muscle power, endurance, and sprint performance. Medicine and Science in Sports and Exercise 34(2):332-343.
- Kockler DR, McCarthy MW, Lawson CL. 2001. Seizure activity and unresponsiveness after hydroxycut ingestion. Pharmacotherapy 21(5):647-651.
- Koshy KM, Griswold E, Schneeberger EE. 1999. Interstitial nephritis in a patient taking creatine. The New England Journal of Medicine 340(10):814-815.
- Kreider RB, Melton C, Rasmussen CJ, Greenwood M, Lancaster S, Cantler EC, Milnor P, Almada AL. 2003. Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Molecular and Cellular Biochemistry 244(1-2):95-104.
- McKenna MJ, Morton J, Selig SE, Snow RJ. 1999. Creatine supplementation increases muscle total creatine but not maximal intermittent exercise performance. Journal of Applied Physiology 87(6):2244-2252
- McRae CA, Agarwal K, Mutimer D, Bassendine MF. 2002. Hepatitis associated with Chinese herbs. European Journal of Gastroenterology and Hepatology 14(5):559-562.
- Nissen SL and Sharp RL. 2003. Effects of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. Journal of Applied Physiology 94(2):651-659.
- Ostojic SM, Ahmetovic Z. 2008. Gastrointestinal distress after creatine supplementation in athletes: are side effects dose dependent? Research in Sports Medicine 16(1):15-22.
- Persky AM, Brazeau GA. 2001. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacological Reviews 53(2):161-176.
- Poortmans JR, Auquier H, Renault V, Durussel A, Saugy M, Brisson GR. 1997. Effect of short-term creatine supplementation on renal responses in men. European Journal of Applied Physiology 76(6):566-567.
- Poortmans JR, Francaux M. 2000. Adverse effects of creatine supplementation: fact or fiction? SportsMedicine 30(3):155-170.
- Poortmans JR, Francaux M. 1999. Long-term oral creatine supplementation does not impair renal function in healthy athletes. Medicine & Science in Sports & Exercise 31(8):1108-1110.
- Powers ME, Arnold BL, Weltman AL, Perrin DH, Mistry D, Kahler DM, Kraemer W, Volek J. 2003. Creatine supplementation increases total body water without altering fluid distribution. Journal of Athletic Training 38(1):44-50.
- Prevost MC, Nelson AG, Morris GS. 1997. Creatine supplementation enhances intermittent work performance. Research Quarterly for Exercise and Sport 68(3):233-240.
- Rawson ES, Clarkson PM, Price TB, Miles MP. 2002. Differential response of muscle phosphocreatine to creatine supplementation in young and old subjects. Acta Physiologica Scandinavica 174(1):57-65.
- Rico-Sanz J, Mendez MTM. 2000. Creatine enhances oxygen uptake and performance during alternating intensity exercise. Medicine and Science in Sports and Exercise 32(2):379-385.
- Robinson TM, Sewell DA, Casey A, Steenge G, Greenhaff PL. 2000. Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. British Journal of Sports Medicine 34(4):284-288.
- Saab G, Marsh GD, Casselman MA, Thompson RT. 2002. Changes in human muscle transverse relaxation following short-term creatine supplementation. Experimental Physiology 87(3):383-389.
- Schilling BK, Stone MH, Utter A, Kearney JT, Johnson M, Coglianese R, Smith L, O'Bryant HS, Fry AC, Starks M, Keith R, Stone ME. 2001. Creatine supplementation and health variables: a retrospective study. Medicine & Science in Sports & Exercise 33(2):183-188.
- Shao A, Hathcock JN. 2006. Risk assessment for creatine monohydrate. Regulatory Toxicology and Pharmacology 45(3):242-251.
- Vahedi K, Domigo V, Amarenco P, Bousser MG. 2000. Ischaemic stroke in a sportsman who consumed MaHuang extract and creatine monohydrate for body building. Journal of Neurology, Neurosurgery, and Psychiatry 68(1):112-113.
- Volek JK, Kraemer WJ, Bush JA, Boetes M, Incledon T, Clark KL, Lynch JM. 1997. Creatine supplementation enhances muscular performance during high-intensity resistance exercise. Journal of the American Dietetic Association 97(7):765-770.
- Volek JS, Mazzetti SA, Farquhar WB, Barnes BR, Gómez AL, Kraemer WJ. 2001. Physiological responses to short-term exercise in the heat after creatine loading. Medicine & Science in Sports & Exercise 33(7):1101-1108.
- Watson G, Casa DJ, Fiala KA, Hile A, Roti MW, Healey JC, Armstrong LE, Maresh CM. 2006. Creatine use and exercise heat tolerance in dehydrated men. Journal of Athletic Training 41(1):18-29.