Anti-Dandruff Products
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
Date
2018-12-07
FOREWORD
This monograph intends to replace the Anti-dandruff Products Monograph dated October 12, 2006.This monograph describes the requirements necessary to receive market authorization [i.e. a Drug Identification Number (DIN) or a Natural Product Number (NPN)], for topical products to be applied on the scalp for the control of dandruff. The monograph identifies the permitted medicinal and non-medicinal ingredients, concentrations, indications, directions and conditions of use for these products to be market authorized without the submission to Health Canada of additional evidence. It may also contain the test methods recommended to be used to comply with the requirements of this monograph. Products which do not meet the criteria outlined in this document can apply for market authorization outside of the monograph stream.
Applicants are reminded that anti-dandruff products, like other non-prescription drugs or natural health products, are subject to the Food and Drug Regulations or the Natural Health Products Regulations administered by the Natural and Non-prescription Health Products Directorate (NNHPD). This includes requirements related to labelling, manufacturing and product specifications. Additional information on labels, outside of those specified in the monograph, such as additional directions for use and/or non-therapeutic claims are acceptable as long as they meet the Guidelines for the Nonprescription and Cosmetic Industry Regarding Non-therapeutic Advertising and Labelling Claims, the Guidelines for Consumer Advertising of Health products for Nonprescription drugs, Natural Health Products, Vaccines and Medical Devices, and are not false, misleading or counterintuitive to the use of the product.
The development of this monograph is the result of a thorough review of existing regulations, guidance documents, policies and current practices within Health Canada and other leading regulatory agencies.
Note
The solidus (/) indicates that the terms and/or the statements are synonymous. Either term or statement may be selected by the applicant. Text in parentheses is additional optional information which can be included on the Product Licence Application form and label at the applicant's discretion.
MEDICINAL INGREDIENT(S)
Anti-dandruff products are classified as natural health products (NHPs) if they contain only (an) ingredient(s) from Table 1. Applicants applying for an NPN can access the appropriate forms and guidance at: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription.html.
Anti-dandruff products are classified as non-prescription drugs if they contain an ingredient from Table 2. Applicants applying for a DIN can access the appropriate forms and templates at: http://hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/index_e.html.
| Proper name(s) 1 | Common name(s) 1 | Source material(s) 1,2 | Quantity 2 |
|---|---|---|---|
| Common name(s) | |||
Table 1 Footnotes
|
|||
2-hydroxybenzoic acid |
Salicylic acid |
Salicylic acid |
1.8 - 3.0% |
Selenium disulfide |
Selenium sulfide |
Selenium sulfide |
0.5 - 2.5% |
Sulfur |
Sulfur |
Sulfur |
2 - 5% |
ROUTE(S) OF ADMINISTRATION
Topical
DOSAGE FORM(S)
Acceptable dosage forms for the age category listed in this monograph and specified route of administration are indicated in the Compendium of Monographs Guidance Document.
USE(S) OR PURPOSE(S)
Self-Care Framework Category I Uses or Purposes:
For products containing ingredients in Table 1 or Table 2:
- (For the relief of) / (Controls) / (Helps prevent) / (Helps prevent [the] recurrence of) / (Reduces [the] recurrence of) / (Reduces) dandruff / (and) (the symptoms of) ([skin / scalp] itching / irritation / redness / flaking [and]/ scaling) (associated with dandruff) (FDA 2007).
DOSE(S)
Subpopulation(s)
Children 2 to 11 years, Adolescents 12 to 17 years, Adults 18 years and older
Quantity(ies)
See Tables 1 and 2.
Permitted Combinations
The permitted combinations are:
- Salicylic acid and sulfur: Salicylic acid: 1.8 - 3.0% + Sulfur: 2 - 5% (Gupta and Nicol 2004)
- Coal tar and dl/l-Menthol (for rinse-off products only): Coal tar solution: 1.8% (w/v) + dl/l-Menthol: 1.5% (FDA 2007)
Directions for use
For products formulated to be applied and rinsed off:
- (Shake well.)
- Apply evenly to scalp, leave on for several minutes, and then rinse off. (Repeat application) (Sweetman 2017).
- Use at least twice per week or as directed by a doctor or pharmacist/ physician or pharmacist/ health care practitioner/ health care provider/ health care professional (FDA 2007).
For products formulated to be applied and left on:
- (Shake well.)
- Apply to scalp one to four times daily or as directed by a doctor or pharmacist/ physician or pharmacist/ health care practitioner/ health care provider/ health care professional (FDA 1991, 2007).
Duration of Use
No statement is required.
RISK INFORMATION
Caution(s) and Warning(s)
For all products:
- For external use only(Sweetman 2017; FDA 2007)
- When using this product avoid contact with eyes. If contact occurs, rinse thoroughly with water. (FDA 2007)
- Stop use and ask/ consult a doctor/ physician/ health care practitioner/ health care provider/ health care professional if condition worsens or does not improve after regular use of this product. (FDA 2007)
- Keep out of reach of children.If swallowed, call a poison control centre or get medical help right away.
For products containing coal tar:
- (When using this product light coloured hair, clothing, and skin may be stained.)
Contraindication(s)
For products containing selenium sulfide or coal tar:
- Do not use within 48 hours of applying hair colours, bleaching agents, tinted / tinting agents, or permanent waving preparations (Krinsky et al. 2017; Sweetman 2017; CTMA 2016; CPMA 2016).
For products containing coal tar:
- Do not use for prolonged periods without asking/ consulting a doctor/ physician/ health care practitioner/ health care provider/ health care professional.
Known Adverse Reaction(s)
No statement required.
NON-MEDICINAL INGREDIENTS
Ingredients must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in that database, the Food and Drug Regulations (FDR), and the current Cosmetic Ingredient Hotlist , when relevant.
STORAGE CONDITION(S)
No statement required.
SPECIFICATIONS
This monograph describes those requirements that are specific to this class of non-prescription drugs and NHPs. Any change to the manufacturing process that impacts the safety and efficacy of the ingredients, such as the use of novel technology (e.g. nanotechnology), requires supporting data and will be reviewed outside the monograph.
For products containing Table 1 NHP medicinal ingredients only:
The finished product specifications must be established in accordance with the requirements described in the NNHPD Quality of Natural Health Products Guide. The medicinal ingredient must comply with the requirements outlined in the NHPID.
For products containing Table 2 non-prescription drug medicinal ingredients:Requirements described in the Regulations to the Food and Drugs Act must be met.
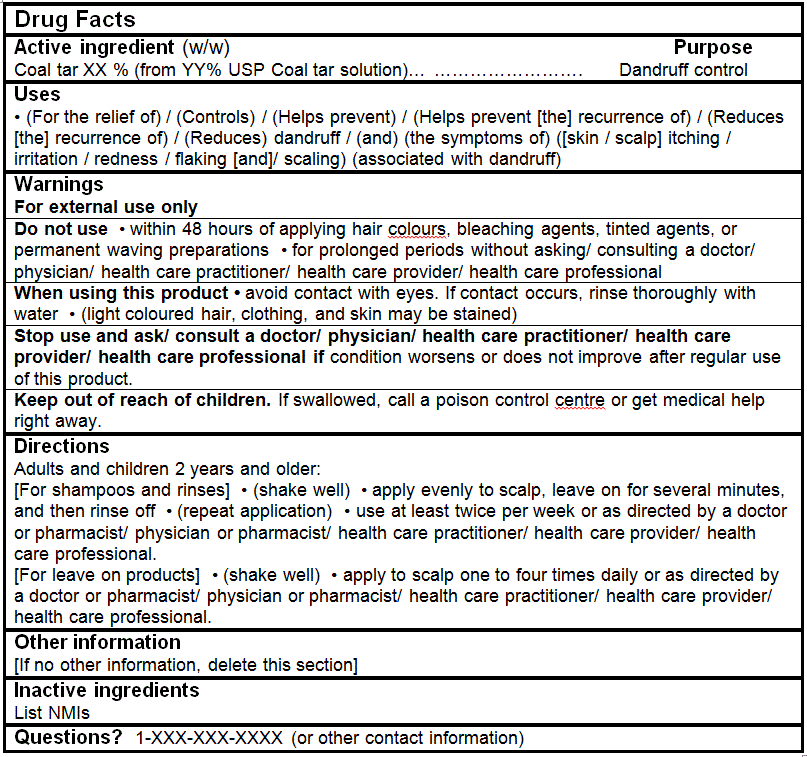
DRUG FACTS TABLES (Format Optional for Self-Care Category I)

References
BP 2018: The British Pharmacopoeia. British Pharmacopoeia Commission. London (UK): Her Majesty's Stationary Office; 2003 2018.
Compendium of products for minor ailments (CPMA). Power B, et all, editors. Canadian Pharmacists Association ; 2016
Compendium of therapeutics for minor ailments (CTMA). Power B et all, editors. Canadian Pharmacists Association ; 2016
FDA 2007: USA Department of Health and Human Services: Food and Drug Administration. 21 CFR 358. Dandruff, seborrheic dermatitis, and psoriasis drug products for over-the-counter human use: final rule; 2007. [Accessed 2018-05-11. Available at: http://www.fda.gov/cder/otcmonographs/Dandruff&Seborrheic_Dermatitis&Psoriasis/dandruff(3 58H).pdf
Guidelines for Consumer Advertising of Health products for Nonprescription drugs, Natural Health Products, Vaccines and Medical Devices. Ad Standards; 2018
Guidelines for the Nonprescription and Cosmetic Industry Regarding Non-therapeutic Advertising and Labelling Claims. Ad Standards;2016.
Gupta AK, Nicol K. The use of sulfur in dermatology. Journal of drugs in dermatology. 2004; 3:427-431.
Health Canada 2015: Quality of Natural Health Products Guide. Health Canada, May 2015. [Accessed 2018 September 27]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/legislation-guidelines/guidance-documents/quality-guide.html
Krinsky DL, Ferreri SP, Hemstreet B, Hume AL, Newton GD, Rollins CJ, Tietze KJ. Handbook of Nonprescription Drugs: An interactive approach for Self-Care, 19th edition. Washington (DC): American Pharmaceutical Association; 2017 (Chapter 34)
Nikitakis J, Lange B, editors. International Cosmetic Ingredient Dictionary and Handbook. 16th edition. Washington (DC): Cosmetic, Toiletry and Fragrance Association; 2016
O'Neil MJ, Smith A, Heckelman PE, Budavari S, editors. Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 2018 Whitehouse Station (NJ): Merck & Co., Inc; 2018.
Sweetman SC, editor. Martindale: The Complete Drug Reference, 39th edition. Grayslake (IL): Pharmaceutical Press; 2002 2017.
USP 41: The United States Pharmacopeia and the National Formulary (USP 41/NF 36). Rockville (MD): United States Pharmacopeial Convention, Inc.; 2018.