WORKOUT SUPPLEMENTS
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization. It is not intended to be a comprehensive review of the medicinal ingredient.
Notes
- Text in parentheses is additional optional information which can be included on the PLA and product label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or statements are synonymous. Either term or statement may be selected by the applicant.
- Sodium and ingredients where sodium is a substantial component (e.g. sodium chloride) are not permitted as a medicinal ingredients on this monograph due to health concerns associated with chronic supplemental use of sodium, namely hypertension, which remains the most common and most important risk factor for cardiovascular disease. However, the use of sodium as a counter-ion in medicinal or non-medicinal ingredients (e.g. sodium salts of minerals) is acceptable where warranted.
Date
November 29, 2024
Proper name(s), Common name(s), Source information
Table 1. Proper name(s), Common name(s), Source information
Group 1: Proteins
| Proper name(s) | Common name(s) | Source information | ||
|---|---|---|---|---|
| Source ingredient(s) | Source material(s) | Part(s) | ||
| Ache ta domesticus | House cricket | N/A | Acheta domesticus | Whole |
| Alfalfa protein concentrate | Alfalfa protein concentrate | N/A | Medicago sativa | Herb top |
| Beef protein isolate | Beef protein isolate | N/A | Bos taurus | Muscle |
| Casein | Casein |
|
Bas taurus | Milk |
|
|
N/A | Bos taurus | Milk |
| Casein micelles | Micellar casein | N/A | Bas taurus | Milk |
| Chia protein concentrate | Chia protein concentrate | N/A | Salvia hispanica | Seed |
| Chia protein isolate1 | Chia protein isolate | N/A | Salvia hispanica | Seed |
| Chickpea protein concentrate | Chickpea protein concentrate | N/A | Cicer arietinum | Seed |
| Chickpea protein isolate1 | Chickpea protein isolate | N/A | Cicer arietinum | Seed |
| Cicer arietinum |
|
N/A | Cicer arietinum | Seed |
| Coconut protein concentrate | Coconut protein concentrate | N/A | Cocos nucifera | Seed endosperm |
| Coconut protein isolate1 | Coconut protein isolate | N/A | Cocos nucifera | Seed endosperm |
| Cucurbita pepo |
|
N/A | Cucurbita pepo | Seed |
| Defatted wheat germ protein | Defatted wheat germ protein | N/A | Triticum aestivum | Seed germ |
| Fava bean protein concentrate | Fava bean protein concentrate | N/A | Vicia faba | Seed |
| Fava bean protein isolate1 | Fava bean protein isolate | N/A | Vicia faba | Seed |
| Fish protein hydrolysate | Fish protein hydrolysate | N/A | Clupea harengus |
|
| Gadus chalcogrammus |
|
|||
| Gadus morhua | Meat | |||
| Merluccius productus | Meat | |||
| Micromesistius poutassou |
|
|||
| Molva dypterygia | Meat | |||
| Salmo salar | Meat | |||
| Scomber scombrus | Meat | |||
| Flaxseed protein | Flaxseed protein | N/A | Linum usitatissimum | Seed |
| Gryllodes sigillatus | Tropical house cricket | N/A | Gryllodes sigillatus | Whole |
| Hemp protein concentrate | Hemp protein concentrate | N/A | Cannabis sativa | Seed |
| Hemp protein isolate1 | Hemp protein isolate | N/A | Cannabis sativa | Seed |
| Lemna minor | Common duckweed | N/A | Lemna minor | Whole plant |
| Lentil protein concentrate | Lentil protein concentrate | N/A | Lens culinaris | Seed |
| Lens culinaris |
|
N/A | Lens culinaris | Seed |
| Linum usitatissimum |
|
N/A | Linum usitatissimum | Seed |
| Medicago sativa |
|
N/A | Medicago sativa | Herb top |
| Milk protein concentrate | Milk protein concentrate | N/A | Bos taurus |
Milk |
| Milk protein isolate1 | Milk protein isolate | N/A | Bos taurus | Milk |
| Oryza sativa |
|
N/A | Oryza sativa | Seed |
| Pea protein concentrate | Pea protein concentrate | N/A | Pisum sativum | Seed |
| Pea protein isolate1 | Pea protein isolate | N/A | Pisum sativum | Seed |
| Pisum sativum | Pea | N/A | Pisum sativum | Seed |
|
|
N/A | Plukenetia volubilis | Seed |
| Plukenetia volubilis |
|
N/A | Plukenetia volubilis | Seed |
|
|
N/A | Solanum tuberosum | Tuber |
| Pumpkin seed protein concentrate | Pumpkin seed protein concentrate | N/A | Cucurbita pepo | Seed |
| Rice protein concentrate | Rice protein concentrate | N/A | Oryza sativa | Seed |
| Salvia hispanica | Chia | N/A | Salvia hispanica | Seed |
| Soy protein concentrate | Soy protein concentrate | N/A | Glycine max | Seed |
| Soy protein isolate1 | Soy protein isolate | N/A | Glycine max | Seed |
| Vicia faba | Fava bean | N/A | Vicia faba | Seed |
| Water lentil protein concentrate | Water lentil protein concentrate | N/A |
|
Whole |
| Wheat protein isolate1 | Wheat protein isolate | N/A | Triticum aestivum | Seed germ |
| Whey protein concentrate | Whey protein concentrate | N/A |
|
Milk |
| Whey protein hydrolysate | Whey protein hydrolysate | N/A |
|
Milk |
| Whey protein isolate1 | Whey protein isolate | N/A |
|
Milk |
| Wolffia globosa | Asian watermeal | N/A | Wolffia globosa | Whole plant |
1For isolate, the potency information should be equivalent to 90% or more protein on a dry weight basis.
Group 2: Amino acids
Group 2a: Essential amino acids
| Proper name(s) | Common name(s) | Source information |
|---|---|---|
| Source ingredient(s) | ||
|
L-Histidine |
|
|
L-Isoleucine |
|
|
L-Leucine |
|
|
|
|
|
|
|
|
L-Phenylalanine |
|
|
L-Threonine |
|
|
|
L-Tryptophan |
|
L-Valine |
|
Group 2b: Non-essential amino acids
| Proper name(s) | Common name(s) | Source information |
|---|---|---|
| Source ingredient(s) | ||
|
L-Alanine |
|
|
beta-Alanine |
|
|
L-Arginine |
|
|
L-Asparagine | L-Asparagine |
|
L-Aspartic acid |
|
|
|
|
|
L-Cysteine |
|
|
|
|
|
|
|
| Aminoacetic acid | Glycine |
|
|
L-Proline | L-Proline |
|
L-Serine | L-Serine |
|
|
|
Group 3: Carbohydrates
| Proper name(s) | Common name(s) | Source information | ||
|---|---|---|---|---|
| Source ingredient(s) | Source material(s) | Part(s) | ||
| D-Fructose | D-Fructose | N/A | Malus domestica | Fruit |
| D-Fructose | N/A | N/A | ||
| D-Galactose | D-Galactose | D-Galactose | N/A | N/A |
| D-Glucose |
|
|
N/A | N/A |
| 4-O-beta-D-Galactopyranosyl-D-glucose | Lactose | Lactose | N/A | N/A |
| Maltodextrin | Maltodextrin | Maltodextrin | N/A | N/A |
| D-Mannose | D-Mannose | D-Mannose | N/A | N/A |
| Solanum tuberosum1 |
|
N/A | Solanum tuberosum | Tuber |
| Oryza sativa1 |
|
N/A | Oryza sativa | Seed |
| D-Ribose |
|
|
N/A | N/A |
|
|
N/A | Acer saccharum | Sap |
| Beta vulgaris | Root | |||
| Borassus flabellifer | Sap | |||
| Malus domestica | Fruit | |||
| Oryza sativa | Seed | |||
| Saccharum officinarum | Leaf stalk | |||
| Pisum sativum1 | Pea starch | N/A | Pisum sativum | Seed |
| Triticum aestivum1 |
|
N/A | Triticum aestivum | Seed endosperm |
| Zea mays1 |
|
N/A | Zea mays | Seed |
| Zea mays1 | Waxy maize starch | N/A | Zea mays | Seed |
1Starch ingredients should be prepared (e.g., gelatinized) such that carbohydrates are readily absorbable.
Group 4: Ergogenic agents
Group 4a: Non-caffeinated ergogenic agents
| Proper name(s) | Common name(s) | Source information | |||
|---|---|---|---|---|---|
| Source ingredient(s) | Source material(s) | Part(s) | Preparation(s) | ||
| Calcium beta-hydroxy-beta-methylbutyrate |
|
|
N/A | N/A | N/A |
|
|
|
N/A | N/A | N/A |
| N-(Aminoiminomethyl)-N-methylglycine monohydrate | Creatine monohydrate | Creatine monohydrate | N/A | N/A | N/A |
| Eleutherococcus senticosus |
|
N/A | Eleutherococcus senticosus | Root | Dry |
| Panax ginseng |
|
N/A | Panax ginseng |
|
Dry |
Group 4b: Caffeine
| Proper name(s) | Common name(s) | Source information | ||
|---|---|---|---|---|
| Source ingredient(s) | Source material(s) | Part(s) | ||
|
Caffeine |
|
N/A | N/A |
| N/A | Camellia sinensis | Leaf | ||
| N/A |
|
Seed | ||
| N/A | Cola acuminata | Seed | ||
| N/A |
|
Leaf | ||
| N/A | Paullinia cupana | Seed | ||
| N/A | Theobroma cacao | Seed | ||
Group 5: Vitamins and Minerals
| Proper name(s) | Common name(s) | Source information |
|---|---|---|
| As per the current NNHPD Multi-Vitamin/Mineral Supplement monograph | ||
Group 6: Complementary ingredients
| Proper name(s) | Common name(s) | Source information | |||
|---|---|---|---|---|---|
| Source ingredient(s) | Source material(s) | Part(s) | Preparation(s) | ||
|
Agmatine | Agmatine sulfate | N/A | N/A | N/A |
|
|
Deanol bitartrate | N/A | N/A | N/A |
| Malpighia glabra |
|
N/A | Malpighia glabra | Fruit |
|
| Piper nigrum |
|
N/A | Piper nigrum | Fruit | Dry |
| 1-[(2E,4E)-5-(1,3-Benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]piperidine | Piperine | N/A | Piper nigrum | Fruit | N/A |
|
Choline |
|
N/A | N/A | N/A |
| Capsicum annuum |
|
N/A | Capsicum annuum | Fruit | Dry |
| all-trans-Lycopene | Lycopene | N/A | Solanum lycopersicum | Fruit flesh | N/A |
| Lycopene | N/A | N/A | |||
|
L-Ornithine |
|
N/A | N/A | N/A |
| 2-Aminoethanesulfonic acid | Taurine |
|
N/A | N/A | N/A |
Reference: NHPID 2024.
Route of Administration
Oral
Dosage Form(s)
This monograph excludes foods or food-like dosage forms as indicated in the Compendium of Monographs Guidance Document.
Acceptable dosage forms for oral use are indicated in the dosage form drop-down list of the web-based Product Licence Application form for Compendial applications.
Note
Liquids and solutions are not permitted for products containing Creatine monohydrate, due to lack of stability of the finished product (Dash and Sawhney 2002).
Use(s) or Purpose(s)
Products providing at least 2.6 g of protein and/or amino acids from Groups 1, 2a and 2b
- Workout support/supplement
- Athletic support/supplement
- Assists in the building of lean muscle tissue/mass when combined with regular weight/resistance training and a healthy balanced diet (NNHPD 2024).
Products containing at least one ingredient from any of Groups 3 or 4a, at or above the relevant minimum doses indicated in the Dose section below
- Workout support/supplement
- Athletic support/supplement
Products containing at least one ingredient from Group 1, at or above the minimum dose indicated in the Dose section below
- Source of protein for the maintenance of good health (CFIA 2024).
- Source of protein which helps build and repair body tissues (CFIA 2024).
- Source of protein which helps build strong muscles (CFIA 2024).
- Source of amino acids involved in muscle protein synthesis (IOM 2005).
Products containing at least one ingredient from Group 2a, at or above the respective minimum dose indicated in the Dose section below
- Source of (an) essential amino acid(s) for the maintenance of good health (CNF 2024).
- Source of (an) (essential) amino acid(s) involved in muscle protein synthesis (IOM 2005).
Products containing all three of L-leucine, L-isoleucine and L-valine, at or above the respective minimum doses indicated in the Dose section below
Source of branched chain amino acids (BCAAs), which are involved in protein synthesis (IOM 2005).
Products containing at least one ingredient from Group 2b, at or above the respective minimum dose indicated in the Dose section below
Source of (an) (non-essential) amino acid(s) involved in muscle protein synthesis (IOM 2005).
Products containing Beta-Alanine, at or above a minimum dose of 800 mg per single dose and 4.8 g, per day
Increases muscle carnosine levels, a factor in delaying neuromuscular fatigue in intermittent high intensity exercises (Hoffman et al. 2008; Hill et al. 2007; Derave et al. 2007; Harris 2006; Stout et al. 2006).
Products containing L-glutamine, at or above a minimum dose of 5 g, per day
- Helps restore plasma glutamine levels depleted after periods of physical stress (e.g. prolonged exhaustive exercise) (Krzywkowski et al. 2001; Bowtell et al. 1999; Castell and Newsholme 1997).
- Helps to assist in muscle cell repair after exercise (Newsholme et al. 2003; IOM 2005).
Products containing at least one ingredient from Group 3, at or above the minimum dose indicated in the Dose section below
- Source of carbohydrates to support energy production (IOM 2005).
- Source of calories which contributes to weight gain (IOM 2005).
- Helps to maintain performance/promote endurance in extended (greater than 60 min), high intensity exercise (Kerksick et al. 2008).
Products containing at least one ingredient from Group 4a, at or above the relevant minimum doses indicated in the Dose section below
Helps increase physical performance during intensive exercise (NNHPD 2024).
Additionally, the following recommended uses may be indicated for products containing the corresponding medicinal ingredients, at or above the relevant minimum doses indicated in the Dose section below:
Calcium beta-hydroxy-beta-methylbutyrate (CaHMB)
Enhances muscle strength in previously untrained individuals in combination with intense resistance training exercise (NNHPD 2024).
L-Carnitine sourced from L-Carnitine tartrate
- Aids in the muscle recovery process by reducing muscle tissue damage associated with a resistance training regimen (Ho et al. 2010; Spiering et al. 2008; Spiering et al. 2007; Kraemer et al. 2006; Kramer et al. 2003; Volek et al. 2002).
- Helps support muscle tissue repair in individuals involved in resistance training (Ho et al. 2010; Spiering et al. 2008; Spiering et al. 2007; Kraemer et al. 2006; Kramer et al. 2003; Volek et al. 2002).
- Helps improve physical performance when used in conjunction with a training regimen (Wall et al. 2011; Cha et al. 2001; Arenas et al. 1994; Huertas et al. 1992; Arenas et al. 1991; Vecchiet et al. 1990; Marconi et al. 1985).
- Helps delay fatigue during physical activity (Cha et al. 2001; Wall et al. 2011; Karahan et al. 2010).
- Helps support fat metabolism (Stephens et al. 2007; Karlic and Lohninger 2004; Müller et al. 2002).
- Helps support fat oxidation (Wall et al. 2011; Stephens et al. 2007; Wutzke and Lorenz 2004; Müller et al. 2002).
Products containing L-Citrulline at or above a minimum dose of 3 g, per day
L-Citrulline is a precursor of L-Arginine (Ochiai et al. 2012; Waugh et al. 2001).
Products containing L-Citrulline sourced from Citrulline malate at or above a minimum dose of 1.7 g (equivalent to 3 g citrulline malate), per day
Supports an increase in athletic performance in high-intensity anaerobic exercise with short rest period (Perez-Guisado and Jakeman 2010; Bailey et al. 2015; Bendahan et al. 2002).
Creatine monohydrate
- Increases body/(lean)muscle mass/size when used in conjunction with a resistance training regimen (Brose et al. 2003; Bemben et al. 2001; Volek et al. 1999; Vandenberghe et al. 1997).
- Improves strength/power/performance in repetitive bouts of brief, highly-intense physical activity (e.g. sprints, jumping, resistance training) (by increasing muscle/intramuscular creatine/phosphocreatine/energy levels) (Okudan and Gökbel 2005; Brose et al. 2003; Preen et al. 2003; Bemben et al. 2001; Volek et al. 1999; Vandenberghe et al. 1997; Hultman et al. 1996)
Eleuthero/Siberian ginseng
Used in Herbal Medicine to help improve physical performance after periods of physical exertion (Bradley 2006; ESCOP 2003; Hoffmann 2003; Mills and Bone 2000).
Panax ginseng
Used in Herbal Medicine to help enhance physical capacity/performance (in cases of physical stress) (Kim et al. 2005; ESCOP 2003; Gross et al. 2002; WHO 1999; Gross et al. 1995; Sotaniemi et al. 1995; Schepdael 1993).
Products containing Caffeine (Group 4b), at or above the minimum dose indicated in the Dose section below
- Helps (temporarily) to relieve/reduce fatigue, to promote endurance, and to enhance motor performance (Philip et al. 2006; Doherty and Smith 2005; Smith et al. 2005).
- Helps (temporarily) to enhance (physical) energy (Philip et al. 2006; Doherty and Smith 2005; Smith et al. 2005).
- Helps (temporarily) to relieve/reduce fatigue/tiredness (Philip et al. 2006; Doherty and Smith 2005; Smith et al. 2005).
Additional claims
Products containing ingredients from Group 5, at or above the minimum doses indicated in the Dose section below
As per the current NNHPD Multi-Vitamin/Mineral Supplements Monograph.
Notes:
- The above uses can be combined on the product label (e.g. Helps temporarily to relieve fatigue and to assist in muscle cell repair after exercise).
- The terms 'Helps' or 'Helps to' can be used interchangeably on the label.
Uses or Purposes Restrictions
- Claims from the NNHPD Multi-Vitamin/Mineral Supplements Monograph are only acceptable in addition to at least one claim from Groups 1 to 4.
Caffeine rules as part of this monograph (Class I):
- Products providing a total amount of caffeine per day that meets the minimum therapeutic dose (100 mg/day) must indicate a use or purpose associated with caffeine (Group 4b).
- Products containing caffeine from any source either synthetic or isolated, must not:
- indicate any uses or purposes related to cardiovascular/heart health listed in the NNHPD Multi-Vitamin/Mineral Supplements Monograph at any dose.
- indicate any uses or purposes related to the maintenance/support of good/general health at a daily dose of 40 mg or more total caffeine from all sources.
Additional caffeine rules when this monograph is combined with other monographs (Class II and III applications):
- Products containing caffeine from any source (synthetic, isolated or a component of plant material) must not:
- indicate any uses or purposes related to healthy blood pressure or cardiovascular health at any dose of caffeine except if supported by a monograph for a specific caffeine-containing medicinal ingredient such as green coffee bean extract.
- indicate any uses or purposes related to nervine/sedative/relaxation, nor contain a sedative at therapeutic dose, at any dose of caffeine.
- indicate any uses or purposes related to the maintenance/support of good/general health at a daily dose of 40 mg or more total caffeine from all sources.
Dose(s)
Subpopulation(s)
Adults 18 years and older
Quantity(ies)
Note:The minimum doses indicated below must be met only for medicinal ingredients which are directly supporting any indicated uses or purposes, as per the Use(s) or Purpose(s) section above.
| Medicinal Ingredients | Doses - Potency constituents | ||
|---|---|---|---|
| Protein | Isoflavones | ||
| Minimum/day | Maximum/day | Maximum/day | |
| Total amount of protein from Group 1 + amounts of amino acids from Groups 2a and 2b in the product2 | 2.6 g of protein | 90 g of protein | N/A |
| Soy protein concentrate and/or isolate | 2.6 g of protein | 35 g of protein | 30 mg of Aglycone Isoflavone Equivalents (AIE) |
Reference: IOM 2005.
1The potency of protein on an "as is" weight basis is required to be indicated on the Product License Application form and label for each medicinal ingredient from Group 1.
2When combining individual amino acids with protein ingredients, the maximum doses for amino acids in Table 3 and Table 4 apply.
| Medicinal Ingredients | Doses | |
|---|---|---|
| Minimum/day | Maximum/day1 | |
| L-Histidine | 49 mg | 220 mg |
| L-Isoleucine | 66.5 mg | 3.5 g |
| L-Leucine | 147 mg | 7 g |
| L-Lysine | 133 mg | 3 g |
| L-Methionine | 66.5 mg | 1 g |
| L-Phenylalanine | 115.5 mg | 339 mg |
| L-Threonine | 70 mg | 301 mg |
| L-Tryptophan | 17.5 mg | 220 mg |
| L-Valine | 84 mg | 3.5 g |
Reference: Doses: Verhoeven et al. 2009; Guttuso et al. 2008; IOM 2005; Coombes and McNaughton 2000; Bassit et al. 2002; Plaitakis et al. 1988; Berry et al. 1982.
1When combining individual amino acids with protein ingredients, the maximum total quantity (90 g) in Table 1 applies.
| Medicinal Ingredients | Doses | ||
|---|---|---|---|
| Minimum/day | Maximum/day1 | Maximum/single dose | |
| L-Alanine | 181.5 mg | 363 mg | N/A |
| L-Arginine | 208.5 mg | 21 g | 8 g |
| L-Asparagine | 4.6 mg | 93.5 mg | N/A |
| L-Aspartic acid | 325 mg | 1 g | N/A |
| beta-Alanine | 240 mg | 6.4 g | 3.2 g |
| L-Citrulline | 150 mg | 6 g | 3 g |
| L-Cysteine | 50 mg | 1 g | N/A |
| L-Glutamic acid | 750 mg | 1.5 g | N/A |
| L-Glutamine | 342.5 mg | 9 g | N/A |
| Glycine | 160 mg | 1.8 g | N/A |
| L-Proline | 259.5 mg | 519 mg | N/A |
| L-Serine | 175.5 mg | 351 mg | N/A |
| L-Tyrosine | 139 mg | 3.6 g | N/A |
References: Doses: NNHPD 2024; Lenders et al. 2009; IOM 2005; Derave et al. 2007; Hill et al. 2007.
1When combining individual amino acids with protein ingredients, the maximum total quantity (90 g) in Table 1 applies.
| Medicinal Ingredients | Doses | ||
|---|---|---|---|
| Minimum/day | Maximum/day | Maximum/single dose | |
| Combined dose for all ingredients from Group 3 in the product | 6.5 g | 180 g | 45 g |
Reference: Dietitians of Canada 2013
| Medicinal Ingredients | Uses or purposes | Methods of preparation | Doses | ||
|---|---|---|---|---|---|
| Minimum/day | Maximum/day | Maximum/single dose | |||
| Calcium beta- hydroxy-beta-methylbutyrate | Enhances muscle strength in previously untrained individuals in combination with intense resistance training exercise | N/A | 3 g | 6 g | N/A |
| L-Carnitine | Muscle recovery, Muscle tissue repair, Workout support/supplement | N/A | 1 g | 4 g | 2 g |
| Physical performance, Fatigue, Workout support/supplement combined with Physical performance/Fatigue | 2 g | ||||
| Fat metabolism, Fat oxidation | 3 g | ||||
| Eleutherococcus senticosus | Used in Herbal Medicine to help improve physical performance after periods of physical exertion | Dry, Powdered, Non-Standardized Extracts (Dry extract, Tincture, Fluid extract, Decoction, Infusion) | 0.91 g of dried root | 6 g of dried root | N/A |
| Panax ginseng | Used in Herbal Medicine to help enhance physical capacity/performance (in cases of physical stress) | Dry, Powdered, Non-Standardized Extracts (Dry extract, Tincture, Fluid extract, Decoction, Infusion) | 0.5 g of dried root/rootlets | 9 g of dried root/rootlets | N/A |
| Standardized extracts (Dry extract) | 200 mg of extract standardized to 4-7% of total ginsenosides; Not to exceed 9 g of dried root/rootlets per day | 600 mg of extract standardized to 4-7% of total ginsenosides; Not to exceed 9 g of dried root/rootlets per day | N/A | ||
References: Doses: CaHMB: Rowlands and Thomson 2009; Gallagher et al. 2000a,b. Carnitine: Wall et al. 2011; Ho et al. 2010; Spiering et al. 2008; Spiering et al. 2007; Stephens et al. 2007; Kraemer et al. 2006; Karlic and Lohninger 2004; Wutzke and Lorenz 2004; Kraemer et al. 2003; Müller et al. 2002; Volek et al. 2002; Benvenga et al. 2001; Cha et al. 2001; Ahmet et al. 2000; Arenas et al. 1994; Huertas et al. 1992; Arenas et al. 1991; Vecchiet et al. 1990; Harper et al. 1988; Marconi et al. 1985. Eleuthero : Bradley 2006; ESCOP 2003; Hoffmann 2003; Blumenthal et al. 2000; Mills and Bone 2000. Panax ginseng : Vuksan et al. 2008; Reay et al. 2006; Sievenpiper et al. 2006; Reay et al. 2005; Sünram-Lea et al. 2005; Kennedy et al. 2004; ESCOP 2003; Kennedy et al. 2002; Scholey and Kennedy 2002; Engels et al. 2001; Kennedy et al. 2001; Scaglione et al. 2001; Blumenthal et al. 2000; Tetsutani et al. 2000; Engels et al. 1996; Scaglione et al. 1996; Gross et al. 1995; Scaglione et al. 1994; Scaglione et al. 1990; Petkov and Mosharrof 1987; D'Angelo et al. 1986; Soldati and Sticher 1980.
Note: When ‘decoction’ or ‘infusion’ is listed as an acceptable method of preparation, ‘decoction concentrate’ or ‘infusion concentrate’ is also allowed.
| Loading Phase | Maintenance Phase | ||||
|---|---|---|---|---|---|
| Min/day | Max/day | Max/single dose | Min/day | Max/day | |
| Option 1 | 15 g | 20 g | 5 g | 2 g | 5 g |
| Option 2 | 3 g | 5 g | N/A | ||
| Min/day | Max/day | |
|---|---|---|
| Option 3 | 3 g | 5 g |
References for Tables 7 and 8: Okudan and Gökbel 2005; Preen et al. 2003; Bemben et al. 2001; Volek et al. 1999; Vandenberghe et al. 1997; Hultman et al. 1996.
| Medicinal Ingredient | Doses | ||
|---|---|---|---|
| Minimum/day | Maximum/day | Maximum/single dose | |
| Caffeine | 100 mg | 400 mg | 200 mg |
Reference: HC 2022.
1Maximum daily dose of 1000 milligrams from NNHPD Caffeine monograph does not apply for Workout Supplements as this maximum dose is not acceptable for prolonged use.
| Medicinal Ingredients | Doses |
|---|---|
| Vitamins and Minerals | As per the current NNHPD Multi-Vitamin/Mineral Supplements Monograph |
| Medicinal ingredients | Methods of preparation | Doses | |
|---|---|---|---|
| Minimum/day | Maximum/day | ||
| Agmatine | N/A | > 0 mg | 2 g |
| Choline | N/A | > 0 mg | 1 g |
| Capsicum annuum | Dry, Powdered, Non-Standardized Extracts (Dry extract*, Tincture, Fluid Extract, Decoction, Infusion) | > 0 mg dried fruit | 650 mg of dried fruit |
| Deanol | N/A | > 0 mg | 750 mg |
| Lycopene | N/A | > 0 mg | 30 mg |
| L-Ornithine | N/A | > 0 mg | 1.5 g |
Malpighia glabra |
Dry, Powdered, Non-Standardzsed Extracts (Dry extract*, Tincture, Fluid Extract, Decoction, Infusion) |
> 0 mg dried or fresh fruit |
10 g of dried fruit |
| 100 g of fresh fruit | |||
| Piper nigrum | Powdered1 | > 0 mg dried fruit | 25 mg of dried fruit |
| Piperine | N/A | > 0 mg | 14 mg |
| Taurine | N/A | > 0 mg | 3 g |
References: Doses: CNF 2024; NNHPD 2024; Wong et al. 2016; Figueroa et al. 2015; Kenyan et al. 2010; TGA 2007; de Montigny et al. 1979; Marsh and Linnoila 1979; Caraceni et al. 1978; Penovich et al. 1978.
1The method of preparation 'powdered' is defined as a dried and ground preparation (= unextracted).
Note:
- *For Capsicum annuum and Malpighia glabra, solvents allowed for the method of preparation “Non-standardized extracts (Dry extract)” as part of this monograph are ethanol and/or water only.
- When ‘decoction’ or ‘infusion’ is listed as an acceptable method of preparation, ‘decoction concentrate’ or ‘infusion concentrate’ is also allowed.
Direction(s) for use
All products (optional)
Ensure to drink optimal fluid before, during, and after exercise.
Products containing creatine monohydrate and making creatine claims
| Option(s)1 | Direction(s) for use and duration(s) of use |
|---|---|
| Option 1 - loading phase of 15-20 g/day | Start with a loading phase of X g2 per day for 5-7 days and follow with a maintenance phase (Y g2/day) |
| Option 2 - loading phase of 3-5 g/day | Start with a loading phase of X g2 per day for a minimum of 4 weeks and follow with a maintenance phase (Y g2/day) |
| Option 3 - no loading phase | Use for a minimum of 4 weeks. |
Reference: NNHPD 2024.
1If more than one option is listed for a product, they should be separated with 'OR' for clarity.
2The dose in grams can be replaced on the label with the number of dosage unit required to reach the loading dose (X g) and the maintenance dose (Y g) (e.g. X scoop(s); sachet(s); serving(s), etc).
Products containing L-carnitine and making Muscle recovery, Muscle tissue repair, Workout/Athletic support/supplement, Physical performance, or Fatigue claims
Take 2-4 hours prior to exercise (Harper et al. 1988).
Products providing more than 200 mg of caffeine, per day (i.e. to be taken in divided doses)
Wait 3 to 4 hours between each dose
Products containing whey protein
Take a few hours before or after taking other medications or health products (Sweetman 2011; Jung et al. 1997).
Products in powder form
(Thoroughly) Mix product in enough liquid (water, juice, etc.) immediately before consumption.
Products for increasing exercise performance (optional)
Consume 45-90 minutes before exercising (Aragon and Schoenfeld 2013).
Products for repairing body tissues/muscles and restoring plasma glutamine levels (optional)
Consume no later than 90 minutes after exercising (Aragon and Schoenfeld 2013).
Products for endurance based on ingredients from Group 3 (Carbohydrates) (optional)
Consume 30-60 grams of carbohydrates, per hour of high intensity exercise (Saunders et al. 2007; Ivy et al. 2003).
Products containing vitamins and/or minerals
As per the current NNHPD Multi-Vitamin/Mineral Supplements Monograph.
Combinations rules
- All ingredients included in this monograph may be combined together, across all Groups.
- The finished product should not exceed a total amount of piperine (from Piper nigrum (black pepper) and/or isolated piperine)) of 14 mg per day.
- For combination of Whey protein isolate, Whey protein concentrate and Whey protein hydrolysate, consult the NNHPD Whey products monograph.
Duration(s) of Use
Products providing more than 200 mg of agmatine, per day
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 3 weeks (Gilad and Gilad 2014; Kenyan et al. 2010).
Products providing 3 g to 9 g of L-arginine, per day
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 8 weeks if you have a cardiovascular disease (Shao and Hathcock 2008; Sydow et al. 2002; Hambrecht et al. 2000; Clarkson et al.1996; Rector et al. 1996).
Products providing more than 9 g and up to 14 g of L-arginine, per day
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 8 weeks if you have a cardiovascular disease or beyond 6 months if you are healthy (Alexander et al. 2005; De Nicola et al. 1999).
Products providing more than 14 g of L-arginine, per day
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 8 weeks if you have a cardiovascular disease or beyond 12 weeks if you are healthy (Tangphao et al. 1999).
Products providing more than 3 g of L-citrulline, per day
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 8 weeks (Harnden et al. 2023; Xie et al. 2023; Smeets et al. 2022; Viribay et al. 2022; Rhim et al 2020; Behpour et al. 2020; Wong et al. 2016; Figueroa et al. 2015).
Product providing more than 3 g of beta-alanine, per day
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 10 weeks (Derave et al. 2007; Hill et al. 2007).
Products containing deanol
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 4 weeks (de Montigny et al. 1979, Marsh and Linnoila 1979, Caraceni et al. 1978. Penovich et al. 1978).
Products containing eleuthero
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 1 month (ESCOP 2003).
Products containing Panax ginseng
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 3 months (Bradley 2006; Mills and Bone 2005; Blumenthal et al. 2000).
Products containing piperine
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 12 weeks (Lieberman et al. 2005).
Products containing vitamins and/or minerals
As per the current NNHPD Multi-Vitamin/Mineral Supplements Monograph.
Risk Information
Caution(s) and warning(s)
All products (except products containing deanol requiring a contraindication)
Ask a healthcare practitioner/health care provider/health care professional/doctor/physician before use if you are pregnant or breastfeeding.
Products containing milk by-products (such as casein, hydrolyzed casein, casein micelles, whey and milk proteins sourced from Bos taurus milk)
Contains milk by-products.
Products providing more than 30 g total protein and/or amino acids (including beta-alanine), per day
Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have a liver or kidney disorder (Shils et al. 2006).
Products containing 40 mg to 400 mg caffeine, per day
Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have high blood pressure or glaucoma (Cornelis and El-Sohemy 2007, Chandrasekaran et al. 2005, Noordzij et al. 2005, Avisar et al. 2002, Arya et al. 2000, Jee et al. 1999, Creighton and Stanton 1990).
Products providing more than 300 mg of caffeine, per day
Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are attempting to conceive (Nawrot et al. 2003).
Products providing 400 mg caffeine, per day
When using this product avoid taking other health products, foods or beverages that contain caffeine and/or increase blood pressure (FDA 2023; Bui et al. 2006; Bouchard et al. 2005; Haller et al. 2005; NIH 2004; Berardi et al. 2002; Vahedi et al. 2000; Zimmerman 1992).
Products containing CaHMB
Ask a health care practitioner /health care provider/health care professional/doctor/physician before use if you are taking cholesterol medications (Nissen et al. 2000).
Products containing cayenne
- Keep out of reach of children. If overdose or accidental ingestion occurs, call a poison control center immediately (CPS 2008).
- Ask a health care practitioner/health care provider/health care professional/doctor/ physician before use if you have a stomach ulcer or inflammation (Brinker 2010; Bradley 2006; Boon and Smith 2004).
Products containing creatine monohydrate
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have a kidney disorder (Pline and Smith 2005; Pritchard and Kalra 1998).
- When using this product you may gain weight (Volek and Rawson 2004; Bemben et al. 2001; Mihic et al. 2000).
Products providing more than 200 mg of agmatine, per day
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have a mood/affective or psychiatric disorder, diabetes or a cardiovascular disease (Freitas et al. 2016; Nissim et al. 2014; Payandemehr et al. 2013; Piletz et al. 2013; Shopsin 2013; Uzbay et al. 2013; Su et al. 2003).
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are taking antidepressant or opioid analgesic medications (Freitas et al. 2016; Payandemehr et al. 2013; Shopsin 2013; Uzbay et al. 2013; Su et al. 2003).
Products providing more than 0.42 g of L-arginine and/or L-citrulline, per day
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have a cardiovascular disease and are attempting an increase in physical activity (Doutreleau et al. 2010; Doutreleau et al. 2006; Schulman et al. 2006; Nagaya et al. 2001; Bednarz et al. 2000; Ceremuzynski et al. 1997; Rector et al. 1996).
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are taking medication for cardiovascular diseases, erectile dysfunction, and/or blood thinners (Huynh et al. 2002; Parker et al. 2002; Siani et al. 2000; Adams et al. 1995).
Products containing L-tryptophan
Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are taking antidepressants (Boyer 2023; Scotton et al. 2019; Erner 2003).
Products containing L-carnitine
Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have a seizure disorder (CPS 2008).
Products containing deanol
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are taking cholinergic or anticholinergic drugs (NIEHS 2002; de Montigny et al. 1979).
- Ask a health care practitioner/health care provider/health care professional/doctor/physician if you have a psychological disorder (NIEHS 2002; Casey 1979; de Montigny et al. 1979).
Products containing eleuthero
Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have an acute infection (Brinker 2010; Barnes et al. 2007; ESCOP 2003; Mills and Bone 2000).
Products containing Panax ginseng
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have diabetes (Brinker 2010; Vuksan et al. 2008; Seely et al. 2008; Sievenpiper et al. 2006; ESCOP 2003; Tetsutani et al. 2000; Sotaniemi et al. 1995; Chin 1991).
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are taking antidepressant medication, blood thinners or digoxin (Brinker 2010; Lee et al. 2008a; Dasgupta and Reyes 2005; Janetzki and Morreale 1997; Gonzalez-Seijo et al. 1995; Shader and Greenblatt 1988; Jones and Runikis 1987; Shader and Greenblatt 1985).
Products containing piperine
Ask a health care practitioner /health care provider/health care professional/doctor/physician before use if you are taking medications or any other health products, as piperine may alter their effectiveness (Han 2011; Srinivasan 2007; Khajuria et al. 2002; Bano et al. 1991).
Products containing vitamins and/or minerals
As per the current NNHPD Multi-Vitamin/Mineral Supplements Monograph.
Contraindication(s)
Products providing more than 9 g of L-arginine, per day
Do not use if you have had a heart attack/myocardial infarction (Sun et al. 2009; Schulman et al. 2006; Bednarz et al. 2005).
Products containing deanol
Do not use if you are pregnant or breastfeeding (NIEHS 2002).
Products containing eleuthero
Do not use if you have high blood pressure (Brinker 2010; Barnes et al. 2007; Blumenthal et al. 2000; Mills and Bone 2000; McGuffin et al. 1997).
Products containing whey proteins and providing 100 mg or more potassium, per day
Do not use if you are taking other potassium-containing salt-substitutes or supplements (Sweetman 2011).
Products containing vitamins and/or minerals
As per the current NNHPD Multi-Vitamin/Mineral Supplements Monograph.
Known adverse reaction(s)
Products containing beta-alanine
When using this product reduce the dose if flushing, tingling or prickling sensation of the skin occurs (Harris et al. 2006; Hill et al. 2007; Jordan et al. 2010)
Products containing deanol
Stop use and ask a health care practitioner/health care provider/health care professional/doctor/physician if you experience drowsiness, confusion, headache, changes in mood, nausea, or gastrointestinal disorders (NIEHS 2002; Casey 1979; Marsh and Linnoila 1979; de Montigny et al. 1979
Products containing caffeine at any dose
Stop use and ask a health care practitioner/health care provider/health care professional/doctor/physician if hypersensitivity/allergy occurs (Infante et al. 2003; Hinrichs et al. 2002).
Products providing 40 mg to 400 mg caffeine, per day
When using this product diuretic effect may occur.
Products providing 200 mg or more caffeine, per day
Stop use and ask a health care practitioner/health care provider/health care professional/doctor/physician if symptoms such as chest pain and irregular heartbeat occur (Higgins and Babu 2013).
Products providing more than 200 mg of agmatine, per day or more than 9 g of L-arginine, per day or more than 30 g of protein and/or amino acids, per day
When using this product you may experience gastrointestinal discomfort/disturbances (Keynan et al. 2010; Grimble 2007; Evans et al. 2004; Clarkson et al. 1996).
Products containing Panax ginseng
Stop use and ask a health care practitioner/health care provider/health care professional/doctor/physician if you experience insomnia, anxiety or headaches (Lee et al. 2008b; Vuksan et al 2008; de Andrade et al. 2007; Sievenpiper et al. 2006; Coon and Ernst 2002; Ellis and Reddy 2002; Scaglione et al. 2001; Siegel 1979).
Products containing vitamins and/or minerals
As per the current NNHPD Multi-Vitamin/Mineral Supplements Monograph.
Non-medicinal ingredients
Must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in the database.
Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations.
Specifications
- The finished product specifications must be established in accordance with the requirements described in the Natural and Non-prescription Health Products Directorate (NNHPD) Quality of Natural Health Products Guide.
- The medicinal ingredient must comply with the requirements outlined in the NHPID.
Hemp protein, Hemp protein isolate and Hemp seed protein
Must not contain more than 10 parts per million delta-9-Tetrahydrocannabinol (THC), or phytocannabinoids that have been isolated or concentrated. The determination of the THC concentration must take into account the potential to convert delta-9-tetrahydrocannabinolic acid (THCA) to THC. These hemp derivatives must also be compliant with the Industrial Hemp Regulations (IHR). All sources of hemp falling under the IHR are expected to be of an approved cultivar, defined in the IHR as any variety of industrial hemp set out in the List of Approved Cultivars, published by the Government of Canada on its website, as amended from time to time. (HC 2021; GC 2018a,b; HC 2018; GC 2003)
Creatine monohydrate
The finished product and/or raw material specifications must meet the process-related impurity acceptance criteria outlined in the USP Creatine monograph (or any other internationally recognized pharmacopoeia). Note that the NNHPD will accept these process-related impurity acceptance criteria for either the finished product or the raw material; however, the procedures described in the USP Creatine monograph are specific to the testing of creatine monohydrate as raw material. As per the NNHPD Quality of Natural Health Products Guide, if alternate methods are used for testing to meet pharmacopoeial specifications, the relevant pharmacopoeia should be consulted for information on whether or not the alternate methods are considered suitable.
Soy protein concentrate/isolate
For an accurate measure of specific isoflavones in AIE, follow the methods outlined in AOAC 2008.03 (Collison 2008).
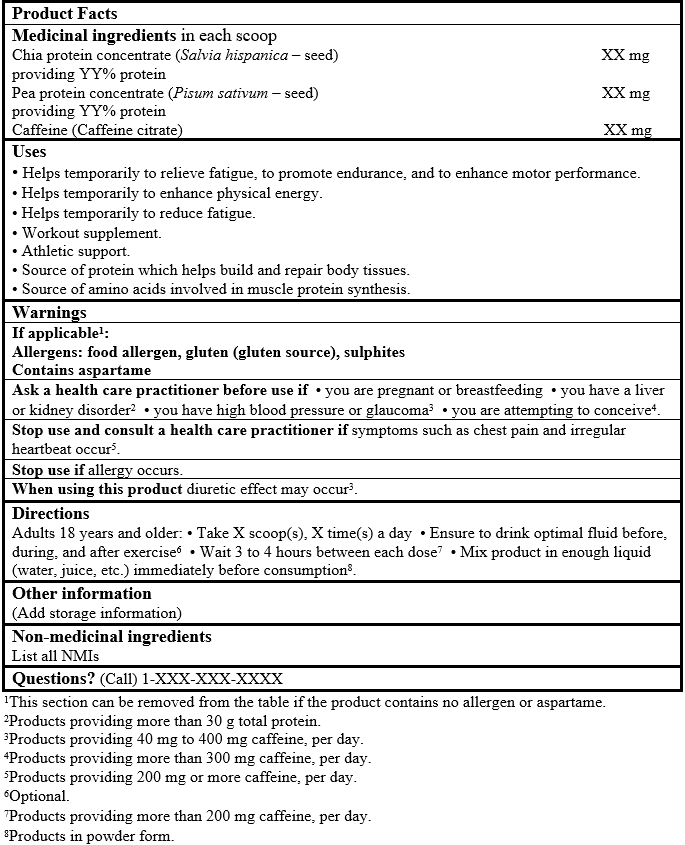
EXAMPLE OF PRODUCT FACTS:
Consult the Guidance Document, Labelling of Natural Health Products for more details.
References Cited
- Adams MR, Forsyth CJ, Jessup W, Robinson J, Celermajer DS. Oral L-arginine inhibits platelet aggregation but does not enhance endothelium-dependent dilation in healthy young men. Journal of the American College of Cardiology 1995;26(4):1054-1061.
- Ahmet U, Abdurrahman K, Sait B, Ahmet E, Salih D, Mendane S, Ates Y, Fatih B, Necmettin K, Kemal D. L-carnitine therapy in non-alcoholic steatohepatitis. Turkish Journal of Pediatrics 2000;11(3):196-201.
- Alexander JW, Metze TJ, McIntosh MJ, Goodman HR, First MR, Munda R, Cardi MA, Austin JN, Goel S, Safdar S, Greenberg N, Chen X, Woodle ES. The influence of immunomodulatory diets on transplant success and complications. Transplantation 2005;79:460-465.
- Aragon AA, Schoenfeld BJ. Nutrient timing revisited: is there a post-exercise anabolic window? Journal of the International Society of Sports Nutrition 2013;10(5).
- Arenas J, Huertas R, Campos Y, Diaz AE, Villalon JM, Vilas E. Effect of L-carnitine on the pyruvate dehydrogenase complex and carnitine palmitoyl transferase activities in muscle of endurance athletes. FEBS Letters 1994;341:91-93.
- Arenas J, Ricoy JR, Encinas AR, Pola P, D'Iddio S, Zeviani M, Didonato S, Corsi M. Carnitine in muscle, serum, and urine of nonprofessional athletes: Effects of physical exercise, training, and L-carnitine administration. Journal of Muscle & Nerve 1991;14(7):598-604.
- Arya LA, Myers DL, Jackson ND. Dietary caffeine intake and the risk for detrusor instability: a case-control study. Obstetrics and Gynecology 2000;96(1):85-89.
- Avisar R, Avisar E, Weinberger D. Effect of coffee consumption on intraocular pressure. The Annals of Pharmacotherapy 2002;36(6):992-995.
- Bailey SJ, Blackwell JR, Lord T, Vanhatalo A, Winyard PG, Jones AM. L-Citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. Journal of Applied Physiology 2015;119(4):385-395.
- Bano G, Raina RK, Zutshi U, Bedi KL, Johri RK, Sharma SC. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. European Journal of Pharmacology 1991;41(6):615-7.
- Barnes J, Anderson LA, Philipson JD. Herbal Medicines, 3rd edition. London (UK): The Pharmaceutical Press; 2007.
- Bassit RA, Sawada LA, Bacurau RFP, Navarro F, Martins Jr E, Santos RVT, Caperuto EC, Rogeri P, Costa Rosa LFBP. Branched-chain amino acid supplementation and the immune response of long-distance athletes. Nutrition 2002;18(5):376-379.
- Bednarz B, Jaxa-Chamiec T, Maciejewski P, Szpajer M, Janik K, Gniot J, Kawka-Urbanek T, Drozdowska D, Gessek J, Laskowski H. Efficacy and safety of oral l-arginine in acute myocardial infarction. Results of the multicenter, randomized, double-blind, placebo-controlled ARAMI pilot trial. Kardiol Pol. 2005;62(5):421-427.
- Bednarz B, Wolk R, Chamiec T, Herbaczynska-Cedro K, Winek D, Ceremuzynski L. Effects of oral L-arginine supplementation on exercise-induced QT dispersion and exercise tolerance in stable angina pectoris. International Journal of Cardiology 2000;75(2-3):205-210.
- Bemben MG, Bemben DA, Loftiss DD, Knehans AW. Creatine supplementation during resistance training in college football athletes. Medicine & Science in Sports & Exercise 2001;33(10):1667-1673.
- Bendahan D, Mattei JP, Ghattas B, Confort-Gouny S, Le Guern ME, Cozzone PJ. Citrulline/malate promotes aerobic energy production in human exercising muscle. British Journal of Sports Medicine 2002;36(4):282-289.
- Benvenga S, Ruggeri RM, Russo A, Lapa D, Campenni A, Trimarchi F. Usefulness of L-carnitine, a naturally occurring peripheral antagonist of thyroid hormone action, in iatrogenic hyperthyroidism: a randomized, double-blind, placebo-controlled clinical trial. Journal of Clinical Endocrinology and Metabolism 2001;86(8):3579-3594.
- Behpour N, Moradi F, Tadibi V. The Effect of Resistance Training Program with Citrulline-Malate on Blood Pressure, Nitric Oxide, and Vascular Endothelial Growth Factor in Postmenopausal Women with Prehypertension. Journal of Fasa University of Medical Sciences 2020;10(1):1913-1922.
- Berardi RR, DeSimone EM, Newton GD, Oszko MA, Popovich NG, Rollins CJ, Shimp LA, Tietze KJ, editors. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care, 13th edition. Washington (DC): American Pharmaceutical Association; 2002.
- Berry HK, Bofinger MK, Hunt MM, Phillips PJ, Guilfoile MB. Reduction of Cerebrospinal Fluid Phenylalanine after Oral Administration of Valine, Isoleucine, and Leucine. Pediatric Research 1982;16:751-755.
- Blumenthal M, Goldberg A, Brinkmann J, editors. Herbal Medicine: Expanded Commission E Monographs. Boston (MA): Integrative Medicine Communications; 2000.
- Boon H, Smith MJ. The Complete Natural Medicine Guide to the 50 Most Common Medicinal Herbs, 2nd edition. Toronto (ON): Robert Rose Inc; 2004.
- Bouchard NC, Howland MA, Greller HA, Hoffman RS, Nelson LS. Ischemic stroke associated with use of an ephedra-free dietary supplement containing synephrine. Mayo Clinic Proceeding 2005;80(4):541-545.
- Bowtell JL, Gelly K, Jackman ML, Patel A, Simeoni M, Rennie MJ. Effect of oral glutamine on whole body carbohydrate storage during recovery from exhaustive exercise. Journal of Applied Physiology 1999;86(6):1770-1777.
- Boyer EW. Serotonin syndrome (serotonin toxicity). UpToDate 2023 [Accessed 2023 October 18]. Available from: https://www.uptodate.com/.
- Bradley PR, editor. British Herbal Compendium: A Handbook of Scientific Information on Widely Used Plant Drugs, Volume 2. Bournemouth (UK): British Herbal Medicine Association; 2006.
- Brinker F. Herb Contraindications and Drug Interactions, 4th edition. Sandy (OR): Eclectic Medical Publications; 2010.
- Brose A, Parise G, Tarnopolsky MA. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. The Journals of Gerontology Series A: Biological Science and Medical Science 2003;58(1):11-19.
- Bui LT, Nguyen DT, Ambrose PJ. Blood pressure and heart rate effects following a single dose of bitter orange. The Annals of Pharmacotherapy 2006;40(1):53-57.
- Caraceni TA, Girotti F, Celano I, Parati E, Balboni L. 2-Dimethylaminoethanol (Deanol) in Huntington's chorea. Journal of Neurology, Neurosurgery and Psychiatry. 1978;41:1114-1118.
- Casey DE. Mood alterations during deanol therapy. Psychopharmacology 1979;62:187-191.
- Castell LM, Newsholme EA. The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition 1997;13(7-8):738-42.
- Ceremuzynski L, Chamiec T, Herbaczynska-Cedro K. Effect of supplemental oral L- arginine on exercise capacity in patients with stable angina pectoris. The American Journal of Cardiology 1997;80(3):331-333.
- CFIA 2024: Canadian Food Inspection Agency. Specific nutrient content claim requirements, Ottawa (ON): Canadian Food Inspection Agency and Health Canada. [Accessed 2024 May 15]. Available from: https://inspection.canada.ca/food-labels/labelling/industry/nutrient-content/specific-claim-requirements/eng/1627085614476/1627085788924
- Cha Y-S, Choi S-K, Suh H, Lee S-N, Cho D, Lim K. Effects of carnitine coingested caffeine on carnitine metabolism endurance capacity in athletes. Journal of Nutritional Science and Vitaminology 2001;47:378-384.
- Chandrasekaran S, Rochtchina E, Mitchell P. Effects of caffeine on intraocular pressure: the Blue Mountains Eye Study. Journal of Glaucoma 2005;14(6):504-507.
- Chin RKH. Ginseng and common pregnancy disorders. Asia-Oceania Journal of Obstetrics and Gynaecology 1991;17(4):379-380.
- Clarkson P, Adams MR, Powe AJ, Donald AE, McCredie R, Robinson J, McCarthy SN, Keech A, Celermajer DS, Deanfield JE. Oral L-arginine endothelium-dependent dilation in hypercholesterolemic young adults. Journal of Clinical Investigation 1996;97(8):1989-1994.
- CNF 2024: Canadian Nutrient File (CNF). Nutrition & Healthy Eating, Food and Nutrition, Health Canada. [Accessed 2024 May 10]. Available from: https://food- nutrition.canada.ca/cnf-fce/serving-portion.do?id=1484
- Collison MW. 2008. Determination of total soy isoflavones in dietary supplements, supplement ingredients, and soy foods by high-performance liquid chromatography with ultraviolet detection: Collaborative Study. Journal of AOAC International 91(3): 489-500.
- Coombes JS, McNaughton LR. Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. The Journal of Sports Medicine and Physical Fitness 2000;40(3):240-246.
- Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Safety 2002;25(5):323-344.
- Cornelis MC, El-Sohemy A. Coffee, caffeine, and coronary heart disease. Current Opinion in Lipidology 2007;18(1):13-19.
- CPS 2008: Compendium of Pharmaceuticals and Specialties: The Canadian Drug Reference for Health Professionals. Ottawa (ON): Canadian Pharmacists Association 2008.
- Creighton SM, Stanton SL. Caffeine: does it affect your bladder? British Journal of Urology 1990;66(6):613-614.
- D'Angelo L, Grimaldi R, Caravaggi M, Marcoli M, Perucca E, Lecchini S, Frigo GM, Crema A. A double-blind, placebo-controlled clinical study on the effect of a standardized ginseng extract on psychomotor performance in healthy volunteers. Journal of Ethnopharmacology 1986;16(1):15-22.
- Dasgupta A, Reyes MA. Effect of Brazillian, Indian, Siberian, Asian, and North American ginseng on serum digoxin measurement by immunoassays and binding of digoxin-like immunoreactive components of ginseng with Fab Fragment of antidigoxin antibody (Digiband). American Journal of Clinical Pathology 2005;124(2):229-236.
- Dash AK, Sawhney A. A simple LC method with UV detection for the analysis of creatine and creatinine and its application to several creatine formulations. Journal of Pharmaceutical and Biomedical Analysis 2002;29(5):939-945.
- de Andrade E, de Masquita AA, de Almeida Claro J, de Andrade PM, Ortiz V, Paranhos M, Srougi M. Study of the efficacy of Korean Red Ginseng in the treatment of erectile dysfunction. Asian Journal of Andrology 2007;9(2):241-244.
- de Montigny C, Chouinard G, Annable L. Ineffectiveness of deanol in tardive dyskinesia: a placebo controlled study. Psychopharmacology 1979;65(3):219-223.
- De Nicola L, Bellizzi V, Minutolo R, Andreucci M, Capuano A, Garibotto G, Corso G, Andreucci VE, Cianciaruso B. Randomized, double-blind, placebo-controlled study of arginine supplementation in chronic renal failure. Kidney International 1999;56:674-684.
- Derave W, Ozdemir MS, Harris RC, Pottier A, Rayngoudt H, Koppo K, Wise JA, Achten E. beta-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. Journal of Applied Physiology 2007;103:1736-1743.
- Dietitians of Canada 2013. Sports Drinks. [Accessed 2024 May 30]. Available from: https://web.archive.org/web/20130508070253/http://www.dietitians.ca/Nutrition-Resources-A-Z/Factsheets/Sports-Nutrition/Sports-Drinks.aspx
- Doherty M, Smith PM. Effects of caffeine ingestion on rating of perceived exertion during and after exercise: a meta-analysis. Scandinavian Journal of Medicine & Science in Sports 2005;15(2):69-78.
- Doutreleau S, Rouyer O, Di Marco P, Lonsdorfer E, Richard R, Piquard F, Geny B. L- Arginine supplementation improves exercise capacity after a heart transplant. American Journal of Clinical Nutrition. 2010;91(5):1261-1267.
- Doutreleau S, Mettauer B, Piquard F, Rouyer O, Schaefer A, Lonsdorfer J., Beny B. Chronic L-Arginine Supplementation Enhances Endurance Exercise Tolerance in Heart Failure Patients. International Journal of Sports Medicine 2006;27(7):567-572.
- Ellis JM, Reddy P. Effects of Panax ginseng on quality of life. The Annals of Pharmacotherapy 2002;36(3):375-379.
- Engels HJ, Kolokouri I, Cieslak TJ, Wirth JC. Effects of ginseng supplementation on supramaximal exercise performance and short-term recovery. Journal of Strength and Conditioning Research 2001;15(3):290-295.
- Engels HJ, Said JM, Wirth JC. Failure of chronic ginseng supplementation to affect work performance and energy metabolism in healthy adult females. Nutrition Research 1996;16(8):1295-1305.
- Erner RA, Meglathery SB, Van Decker WA, Gallagher RM. Seratonin Syndrome and Other Serotonergic Disorders. Pain Medicine 2003;4(1):63-74.
- ESCOP 2003: ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products, 2nd edition. Exeter (UK): European Scientific Cooperative on Phytotherapy and Thieme; 2003.
- Evans WR, Fernstrom JD, Thompson J, Morris SM Jr, Kuller LH. 2004. Biochemical responses of healthy subjects during dietary supplementation with L-arginine. Journal of Nutritional Biochemistry 15(9):534-539.
- FDA 2023: Food and Drug Administration. Title 21 Chapter 1 Part 340. Stimulant drug products for over-the-counter human use. Washington (DC): U.S. Food and Drug Administration, Department of Health and Human Services; 2023. [Accessed 2023 August 28]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=340&showFR=1
- Figueroa A, Alvarez-Alvarado S, Ormsbee MJ, Madzima TA, Campbell JC, Wong A. Impact of L-citrulline supplementation and whole-body vibration training on arterial stiffness and leg muscle function in obese postmenopausal women with high blood pressure. Experimental Gerontology 2015;63:35-40.
- Freitas AE, Neis VB, Rodrigues ALS Agmatine, a potential novel therapeutic strategy for depression. European Neuropsychopharmacology 2016;26:1885-1899.
- Gallagher PM, Carrithers JA, Godard MP, Schulze KE, Trappe SW. Beta-hydroxy-beta- methylbutyrate ingestion, Part I: effects on strength and fat free mass Medicine & Science in Sports & Exercise 2000 Dec;32(12):2109-15a.
- Gallagher PM, Carrithers JA, Godard MP, Schulze KE, Trappe SW. Beta-hydroxy-beta- methylbutyrate ingestion, part II: effects on hematology, hepatic and renal function. Medicine & Science in Sports and Exercise 2000 Dec;32(12):2116-9b.
- GC 2018a. Government of Canada. Cannabis Act. Last amended on April 27, 2023 [Accessed 2024 May 29]. Available at: https://laws-lois.justice.gc.ca/eng/acts/C-24.5/
- GC 2018b. Government of Canada. Industrial Hemp Regulations. Last amended on January 15, 2019 [Accessed 2024 May 29]. Available at: https://laws-lois.justice.gc.ca/eng/regulations/SOR-2018-145/
- GC 2003. Government of Canada. Natural Health Products Regulations. Last amended on November 24, 2023 [Accessed 2024 May 29]. Available at: https://laws-lois.justice.gc.ca/eng/regulations/sor-2003-196/
- Gilad GM, Gilad VH. Long-term (5 years), high dosage of dietary agmatine - Evidence of safety: A case report. Journal of Medicinal Food 2014;17(11):1256-1259.
- Gonzalez-Seijo JC, Ramos YM, Lastra I. Manic episode and ginseng: report of a possible case. Journal of Clinical Psychopharmacology 1995;15(6):447-448.
- Grimble GK. Adverse gastrointestinal effects of arginine and related amino acids. The Journal of Nutrition 2007;137(6 Suppl 2):1693S-1701S.
- Gross D, Krieger D, Efrat R, Dayan. Ginseng extract G115 for the treatment of chronic respiratory diseases. Schweiz Zschr Ganzheits Medizin 1995;1:29-33.
- Gross D, Shenkman Z, Bleiberg B, Dayan M, Gittelson M, Efrat R. Ginseng improves pulmonary functions and exercise capacity in patients with COPD. Monaldi Archives for Chest Disease 2002;57(5-6):242-246.
- Guttuso T, McDermott MP, Su H, Kieburtz K Effects of L-isoleucine and L-valine on hot flushes and serum homocysteine. Obstetrics & Gynecology 2008;112(1): 109-115.
- Haller CA, Benowitz NL, Jacob P. Hemodynamic effects of ephedra-free weight-loss supplements in humans. The American Journal of Medicine 2005;118(9):998-1003.
- Hambrecht R, Hilbrich L, Erbs S, Gielen S, Fiehn E, Schoene N, Schuler. Correction of endothelial dysfunction in chronic heart failure: additional effects of exercise training and oral L- arginine supplementation. Journal of the American College of Cardiology 2000;35(3):706-713.
- Han HK. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert Opinion on Drug Metabolism & Toxicology 2011;7(6):721-729.
- Harnden CS, Agu JO, Gascoyne TO. Effects of citrulline on endurance performance in young healthy adults: a systematic review and meta-analysis. Journal of the International Society of Sports Nutrition. 2023 Dec 31;20(1):2209056.
- Harper P, Elwin CE, Cederblad G. Pharmacokinetics of intravenous and oral bolus doses of Lcarnitine in healthy subjects. European Journal of Clinical Pharmacology 1988;35:555-562.
- Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006 May;30(3):279-289.
- HC 2022: Health Canada. Caffeine in foods. Health Canada; 2022. [Accessed 2024 May 29]. Available from: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/caffeine-foods.html
- HC 2021. Health Canada. Prescription Drug List. [Accessed 2024 May 29]. Available from: https://hpr-rps.hres.ca/pdl.php?lang=en
- HC 2018. Health Canada. Health products containing cannabis or for use with cannabis: Guidance for the Cannabis Act, the Food and Drugs Act, and related regulations. [Accessed 2024 May 29]. Available from: https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/cannabis-health-products-guidance-eng(2).pdf
- Higgins JP, Babu KM, Caffeine reduces myocardial blood flow during exercise. The American Journal of Medicine 2013;126(8):730-e 1-8.
- Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA. Influence of β- alanine supplementation on skeletal muscle carnosine concentrations and high intensity muscle capacity. Amino Acids 2007;32:225-233.
- Hinrichs R, Hunzelmann N, Ritzkowsky A, Zollner TM, Krieg T, Scharffetter-Kochanek K. Caffeine hypersensitivity. Allergy 2002;57(9):859-60.
- Ho JY, Kraemer WJ, Volek JS, Fragala MS, Thomas GA, Dunn-Lewis C, Coday M, Hakkinen K, Maresh CM. L-carnitine L-tartrate supplementation favorably affects biochemical markers of recovery from physical exertion in middle-aged men and women. Metabolism Clinical and Experimental Journal 2010;59:1190-1199.
- Hoffman JR, Ratamess NA, Faigenbaum AD, Ross R, Kang J, Stout JR, Wise JA. Short-term duration beta-alanine supplementation increases training volume and reduces subjective feelings of fatigue in college football players. Nutrition Research 2008;28(1):31-35.
- Hoffmann D. Medical Herbalism. Rochester (VT): Healing Arts Press; 2003.
- Huertas R, Campos Y, Diaz E, Esteban J, Vechietto L, Montanari G, D'Iddio S, Corsi M, Arenas J. Respiratory chain enzymes in muscle of endurance athletes: Effect of L-carnitine. Biochemical and biophysical research communications 1992;188(1):102-107.
- Hultman E, Söderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. Journal of Applied Physiology 1996;81(l):232-237.
- Huynh NT, Tayek JA. Oral arginine reduces systemic blood pressure in type 2 diabetes: Its potential role in nitric oxide generation. American College of Nutrition 2002;21(5):422-427.
- Infante S, Baeza ML, Calvo M, De Barrio M, Rubio M, Herrero T. Anaphylaxis due to caffeine. Allergy 2003;58(7):681-682.
- IOM: Institute of Medicine of the National Academies. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Food and Nutrition Board. Washington D.C. The National Academies Press; 2005.
- Ivy JL, Res PT, Sprague RC, Widzer MO. Effect of a Carbohydrate-Protein Supplement on Endurance Performance During Exercise of Varying Intensity. International Journal of Sport Nutrition and Exercise Metabolism 2003;13:388-401.
- Janetzki K, Morreale AP. Probable interaction between warfarin and ginseng. American Journal of Health-System Pharmacy 1997;54(6):692-693.
- Jee SH, He J, Whelton PK, Suh II, Klag MJ. The effect of chronic coffee drinking on blood pressure: a meta-analysis of controlled clinical trials. Hypertension 1999;33(2):647-652.
- Jones BD, Runikis AM. Interaction of ginseng with phenelzine. Journal of Clinical Pharmacology 1987;7(3):201-202.
- Jordan T, Lukaszuk J, Misic M, Umoren J. Effect of beta-alanine supplementation on the onset of blood lactate accumulation (OBLA) during treadmill running: Pre/post 2 treatment experimental design. Journal of the International Society for Sports Nutrition 2010 May 19;7:20.
- Jung H, Peregrina AA, Rodriguez JM, Moreno-Esparza R. The influence of coffee with milk and tea with milk on the bioavailability of tetracycline. Biopharmaceutics & Drug Disposition 1997;18(5):459-463.
- Karahan M, Coksevim B, Artis S. The effect of L-carnitine supplementation on 1500 m running performance. Science, Movement and Health Journal 2010;10(2):504-507.
- Karlic H, Lohninger A. Supplementation of L-carnitine in athletes: Does it make sense? Journal of Nutrition 2004;20:709-715.
- Kennedy DO, Haskell CF, Wesnes KA, Scholey AB. Improved cognitive performance in human volunteers following administration of guarana (Paullinia cupana) extract: comparison and interaction with Panax ginseng. Pharmacology, Biochemistry and Behavior 2004;79(3):401-411.
- Kennedy DO, Scholey AB, Wesnes KA. Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng, and a ginkgo/ginseng combination to healthy young adults. Physiology & Behavior 2002;75(5):739-751.
- Kennedy DO, Scholey AB, Wesnes KA. Dose dependent changes in cognitive performance and mood following acute administration of Ginseng to healthy young volunteers. Nutritional Neuroscience 2001;4(4):295-310.
- Kerksick C, Harvey T, Stout J. Campbell B, Wilborn C, Kreider R, Kalman D, Ziegenfuss T, Lopez H, Landis J, Ivy JL, Antonio J. International Society of Sports Nutrition position stand: nutrient timing. Journal of The International Society of Sports Nutrition 2008;5:17.
- Keynan O, Mirovsky Y, Dekel S, Gilad VH, Gilad GM. Safety and efficacy of dietary agmatine sulfate in lumbar disc-associated radiculopathy. An open-label, dose-escalating study followed by a randomized, double-blind, placebo-controlled trial. Pain Medicine 2010;11:356-368.
- Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine 2002;9(3):224-31.
- Kim SH, Park KS, Chang MJ, Sung JH. Effects of panax ginseng extract on exercise-induced oxidative stress. Journal of Sports Medicine and Physical Fitness 2005;45(2):178-182.
- Kraemer WJ, Spiering BA, Volek JS, Ratamess NA, Sharman MJ, Rubin MR, French DN, Silvestre R, Hatfield DL, Van Heest JC, Vingren JL, Judelson DA, Deschenes MR, Maresh CM. Androgenic responses to resistance exercise: Effects of feeding and L-Carnitine. Official Journal of the American College of Sports Medicine 2006;1288-1296.
- Kraemer WJ, Volek JS, French DN, Rubin MR, Sharman MJ, Gomez AL, Ratamess NA, Newton RU, Jemiolo B, Craig BW, Hakkinen K. The effects of L-carnitine L-tartrate supplementation on hormonal responses to resistance exercise and recovery. Journal of Strenght and Conditioning Research 2003;17(3):455-462.
- Krzywkowski K, Petersen EW, Ostrowski K, Link-Amster H, Boza J, Halkjaer-Kristensen J, Pedersen BK. Effect of glutamine and protein supplementation on exercise-induced decreases in salivary IgA. Journal of Applied Physiology 2001;91(2):832-838.
- Lee SH, Ahn YM, Ahn SY, Doo HK, Lee BC. Interaction between warfarin and Panax ginseng in ischemic stroke patients. The Journal of Alternative and Complementary Medicine 2008a;14(6):715-721.
- Lee ST, Chu K, Sim JY, Heo JH, Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Disease and Associated Disorders 2008b;22(3):222-226.
- Lenders CM, Liu S, Wilmore DW, Sampson L, Dougherty LW, Spiegelman D, Willett WC. Evaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing data. European Journal of Clinical Nutrition 2009;63(12):1433-1439.
- Lieberman S, Spahrs R, Stanton A. Martinez L, Grinder M. 2005. Weight loss, body measurements, and compliance: A 12-week total lifestyle intervention pilot study. Alternative & Complementary Therapies 2005;11(6):307-313.
- Marconi C, Sassi G, Carpinelli A, Cerretelli P. Effect of L-carnitine loading on the aerobic and anaerobic performance of endurance athletes. European Journal of Applied Physiology 1985;54:131-135.
- Marsh GR, Linnoila M. The effects of deanol on cognitive performance and electrophysiology in elderly humans. Pharmacology 1979;66(1):99-104.
- McGuffin M, Hobbs C, Upton R, Goldberg A, editors. American Herbal Products Association's Botanical Safety Handbook. Boca Raton (FL): CRC Press; 1997.
- Mihic S, MacDonald JR, McKenzie S, Tarnopolsky MA. Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Medicine & Science in Sports and Exercise 2000;32(2):291-296.
- Mills S, Bone K. 2005. The Essential Guide to Herbal Safety. St. Louis (MO): Elsevier Churchill Livingstone.
- Mills S, Bone K. 2000. Principles and Practice of Phytotherapy. Toronto (ON): Churchill Livingstone.
- Müeller DM, Seim H, Kiess W, Löster H, Richter T. Effects of oral L-carnitine supplementation on in vivo long-chain fatty acid oxidation in healthy adults. Journal of Metabolism 2002;51(11):1389-1391.
- Nagaya N, Uematsu M, Oya H, Sato N, Sakamaki F, Kyotani S, Ueno K, Nakanishi N, Yamagishi M, Miyatake K. Short-term oral administration of L-arginine improves hemodynamics and exercise capacity in patients with precapillary pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine 2001;163(4):887-891.
- Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M. Effects of caffeine on human health. Food Additives and Contaminants 2003;20(1):1-30.
- Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate-their central role in cell metabolism and function. Cell Biochemistry and Function 2003;21(1):1-9.
- NHPID 2024: Natural Health Products Ingredients Database. Natural and Non-Prescription Health Products Directorate. [Accessed 2024 May 10]. Available from: http://webprod.hc- sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do
- NIEHS 2002: National Institute of Environmental Health Sciences - Dimethylethanolamine (DMAE) and selected salts and esters: Review of toxicological literature. [Accessed 2023 October 17]. Available from: https://ntp.niehs.nih.gov/sites/default/files/ntp/htdocs/chem_background/exsumpdf/dmae_update_110002_508.pdf
- NIH 2004: National Institutes of Health. Office of Dietary Supplements. Fact sheets. Ephedra and ephedrine alkaloids for weight loss and athletic performance; 2004. [Accessed 2023 October 16]. Available from: https://ods.od.nih.gov/factsheets/ephedraandephedrine-HealthProfessional/.
- Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, Fuller J. B-Hydroxy-B- Methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr 2000;130:1937-1945.
- Nissim I, Horyn O, Daikhin Y, Chen P, Li C, Wehrli SL, Nissim I, Yudkoff M. The molecular and metabolic influence of long term agmatine consumption. Journal of Biological Chemistry 2014;289(14):9710-9729.
- NNHPD 2024: Natural and Non-Prescription Health Products Directorate. Internal evidence on NHPs and current NNHPD's monographs.
- Noordzij M, Uiterwaal CS, Arends LR, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to chronic intake of coffee and caffeine: a meta-analysis of randomized controlled trials. Journal of Hypertension 2005;23(5):921-928.
- Ochiai M, Hayashi T, Morita M, Ina K, Maeda M, Watanebe F, Morishita K. Short-term effects of L-citrulline supplementation on arterial stiffness in middle-aged men. International Journal of Cardiology 2012;155(2):257-261.
- Okudan N, Gökbel H. The effects of creatine supplementation on performance during the repeated bouts of supramaximal exercise. Journal of Sports Medicine and Physical Fitness 2005;45(4):507-512.
- Parker JO, Parker JD, Caldwell RW, Farrell B, Kaesemeyer WH. The effect of supplemental L-arginine on tolerance development during continuous transdermal nitroglycerin therapy. Journal of the American College of Cardiology 2002;39(7):1199-1203.
- Payandemehr B, Rahimian R, Bahremand A, Ebrahimi A, Saadat S, Moghaddas P, Fadakar K, Derakhshaniam H, Dehpour AR. Role of nitric oxide in additive anticonvulsant effects of agmatine and morphine. Physiology & Behavior 2013;118:52-57.
- Penovich P, Morgan JP, Kerzner B, Karch F, Goldblatt D. Double-blind evaluation of deanol in tardive dyskinesia. Journal of American Medical Association. 1978;239(19):1997-1998.
- Perez-Guisado J, Jakeman PM. Citrulline malate enhances athletic anaerobic performance and relieves muscle soreness. The Journal of Strength & Conditioning Research 2010;24(5):1215-1222.
- Petkov VD, Mosharrof AH.. Effects of standardized ginseng extract on learning, memory and physical capabilities. American Journal of Chinese Medicine 1987;15(1-2):19-29.
- Philip P, Taillard J, Moore N, Delord S, Valtat C, Sagaspe P, Bioulac B. The effects of coffee and napping on night time highway driving: a randomized trial. Annals of Internal Medicine 2006;144(11):785-791.
- Piletz JE, Aricioglu F, Cheng J-T, Fairbanks CA, Gilad V, Haenisch G, Halaris A, Hung S, Lee JE, Li J, Liu P, Molderings GJ, Lucia A, Rodrigues S, Satriano J, Seong GJ, Wilcox G, Wu N, Gilad GM. Agmatine: clinical applications after100 years in translation. Drug Discovery Today 2013;18(17):880-893.
- Plaitakis A, Smith J, Mandeli J, Yahr MD. Pilot trial of branched-chain amino acids in amyotrophic lateral sclerosis. The Lancet 1988;1(8593):1015-1018.
- Pline KA, Smith CL. The effect of creatine intake on renal function. The Annals of Pharmacotherapy 2005;39(6):1093-1096.
- Preen D, Dawson B, Goodman C, Beilby J, Ching S. Creatine supplementation: a comparison of loading and maintenance protocols on creatine uptake by human skeletal muscle. International Journal of Sport Nutrition and Exercise Metabolism 2003;13(1):97-111.
- Pritchard NR, Kalra PA. Renal dysfunction accompanying oral creatine supplements. The Lancet 1998;351(9111):1252-1253.
- Reay JL, Kennedy DO, Scholey AB. Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained 'mentally demanding' tasks. Journal of Psychopharmacology 2006;20(6):771-781.
- Reay JL, Kennedy DO, Scholey AB. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. Journal of Psychopharmacology 2005;19(4):357-365.
- Rector TS, Bank AJ, Mullen KA, Tschumperlin LK, Sih R, Pillai K, Kubo SH. Randomized, double-blind, placebo-controlled study of supplemental oral -arginine in patients with heart failure. Circulation 1996;93(12):2135-2141.
- Rhim HC, Kim SJ, Park J, Jang KM. Effect of citrulline on post-exercise rating of perceived exertion, muscle soreness, and blood lactate levels: A systematic review and meta-analysis. Journal of Sport and Health Science. 2020 Dec 1;9(6):553-561.
- Rowlands DS, Thomson JS. Effects of beta-hydroxy-beta-methylbutyrate supplementation during resistance training on strength, body composition, and muscle damage in trained and untrained young men: a meta-analysis. Journal of Strength & Conditioning Research 2009 May;23(3):836-846.
- Saunders MJ. Coingestion of Carbohydrate-Protein during endurance exercise: influence on performance and recovery. International Journal of Sport Nutrition and Exercise Metabolism 2007;17:S87-S103.
- Scaglione F, Weiser K, Alessandria M. Effects of the standardised ginseng extract G115 in patients with chronic bronchitis. Clinical Drug Investigation 2001;21(1):41-45.
- Scaglione F, Cattaneo G, Alessandria M, Cogo R. Efficacy and safety of the standardized ginseng extract G115 for potentiating vaccination against common cold and/or influenza syndrome. Drugs Under Experimental and Clinical Research 1996;22(2):65-72.
- Scaglione F, Cogo R, Cocuzza C, Arcidiacono M, Beretta A. Immunomodulatory effects of Panax ginseng C.A. Meyer (G115) on alveolar macrophages from patients suffering with chronic bronchitis. International Journal of Immunotherapy 1994;10(1):21-24.
- Scaglione F, Ferrara F, Dugnani S, Falchi M, Santoro G, Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Under Experimental and Clinical Research 1990;16(10):537-542.
- Schepdael PV. Les effets du ginseng G115 sur la capacité physique de sportifs d'endurance. Acta Therapeutica 1993;19(4):337-347.
- Scholey AB, Kennedy DO. Acute, dose-dependent cognitive effects of Ginkgo biloba, Panax ginseng and their combination in healthy young volunteers: differential interactions with cognitive demand. Human Psychopharmacology 2002;17(1):35-44.
- Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A, Hare JM, Gerstenblith G. L-Arginine therapy in acute myocardial infarction: the vascular interaction with age in myocardial infarction (VINTAGE MI) randomised clinical trial. Journal of American Medical Association 2006;295(1):58-64.
- Scotton WJ, Hill LJ, Barnes NM. Serotonin syndrome: pathophysiology, clinical features, management, and potential future directions. International Journal of Tryptophan Research 2019;12:1178646919873925.
- Seely D, Dugoua JJ, Perri D, Mills E, Koren G. Safety and efficacy of Panax ginseng during pregnancy and lactation. The Canadian Journal of Clinical Pharmacology 2008;15(1):e87-e94.
- Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. Journal of Clinical Psychopharmacology 1988;8(4):235.
- Shader RI, Greenblatt DJ. Phenelzine and the dream machine - ramblings and reflections. Journal of Clinical Psychopharmacology 1985;5(2):65.
- Shao A, Hathcock JN. Risk assessment for the amino acids taurine, L-glutamine and Larginine. Regulatory Toxicology and Pharmacology 2008;50:376-399.
- Shils ME, Olson JA, Shike M, Ross AC, editors. Modern Nutrition in Health and Disease, 10th edition. Philadelphia (PA): Lippincott Williams and Wilkins; 2006.
- Shopsin B. The clinical antidepressant effect of exogenous agmatine is not reversed by parachlorophenylalanine: A pilot study. Acta Neuropsychiatrica 2013;25(2):113-118.
- Siani A, Pagano E, Iacone R, Iacoviello L, Scopacasa F, Strazzullo P. Blood pressure and metabolic changes during dietary L-arginine supplementation in humans. American Journal of Hypertension 2000;13(5):547-551.
- Siegel RK. Ginseng abuse syndrome. Problems with the panacea. The Journal of the American Medical Association 1979;241(15):1614-1615.
- Sievenpiper JL, Sung MK, Buono MD, Seung-Lee K, Nam KY, Arnason JT, Leiter LA, Vuksan V. Korean red ginseng rootlets decrease acute postprandial glycemia: results from sequential preparation- and dose-finding studies. Journal of the American College of Nutrition 2006;25(2):100-107.
- Smeets ET, Mensink RP, Joris PJ. Effects of l-citrulline supplementation and watermelon consumption on longer-term and postprandial vascular function and cardiometabolic risk markers: a meta-analysis of randomised controlled trials in adults. British Journal of Nutrition. 2022 Nov;128(9):1758-1770.
- Smith A, Sutherland D, Christopher G. Effects of repeated doses of caffeine on mood and performance of alert and fatigued volunteers. Journal of Psychopharmacology 2005;19(6):620- 626.
- Soldati F, Sticher O. HPLC separation and quantitative determination of ginsenosides from Panax ginseng, Panax quinquefolium and from ginseng drug preparations. 2e communication. Planta Medica 1980;39(4):348-357.
- Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care 1995;18(10):1373-1375.
- Spiering BA, Kraemer WJ, Hatfield DL, Vingren JL, Fragala MS, Ho J-Y, Thomas GA, Hakkinen K, Volek JS. Effects of L-carnitine L-tartrate supplementation on muscle oxygenation responses to resistance exercise. Journal of Strength and Conditioning Research 2008;22(4):1130-1135.
- Spiering BA, Kraemer WJ, Vingren JL, Hatfield DL, Fragala MS, Ho J-Y, Maresh CM, Anderson JM, Volek JS. Responses of criterion variables to different supplemental doses of Lcarnitine L-tartrate. Journal of Strength and Conditioning Research 2007;21:259-264.
- Srinivasan K. Black Pepper and its pungent principle-piperine: a review of diverse physiological effects. Critical Reviews in Food Science and Nutrition 2007;47(8):735-748.
- Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. Journal of Physiology 2007;581(2):431-444.
- Stout JR, Cramer JT, Mielke M, O'Kroy J, Torok DJ, Zoeller RF. Effects of twenty-eight days of beta-alanine and creatine monohydrate supplementation on the physical working capacity at neuromuscular fatigue threshold. Journal of Strength and Conditioning Research 2006;20(4):928-931.
- Su R-B, Li J, Qin B-Y A biphasic opioid function modulator: agmatine. Acta Pharmaceutica Sinica 2003;24(7):631-636.
- Sun T, Zhou WB, Luo XP, Tang YL, Shi HM. 2009. Oral L-arginine supplementation in acute myocardial infarction therapy: a meta-analysis of randomized controlled trials. Clin Cardiol. 32(11):649-52.
- Sünram-Lea SI, Birchall RJ, Wesnes KA, Petrini O. The effect of acute administration of 400 mg of Panax ginseng on cognitive performance and mood in healthy young volunteers. Current Topics in Nutraceutical Research 2005;3(1):65-74.
- Sweetman SC, editor. 2011. Martindale: The Complete Drug Reference, 37th edition. London (GB): Pharmaceutical Press.
- Sydow K, Schwedhelm E, Arakawa N, Bode-Böger SM, Tsikas D, Hornig B, Frölich, Böger RH. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovascular Research 2003;57(1):244-252.
- Tangphao O, Chalon S, Moreno HJr, Hoffman BB, Blaschke TF. Pharmacokinetics of L-arginine during chronic administration to patients with hypercholesterolaemia. Clinical Science 1999;96:199-207.
- Tetsutani T, Yamamura M, Yamaguchi T, Onoyama O, Kono M. Can red ginseng control blood glucose in diabetic patients. The Ginseng Review 2000;28:44-47.
- TGA 2007: Australian Therapeutic Goods Administration. CMEC 64: Complementary Medicines Evaluation Committee, Extracted Ratified Minutes Sixty-fourth Meeting, 14 December 2007. Australian Government Department of Health and Aging, Sydney, Australia;2007. [Accessed 2023 October 17]. Available from: https://www.tga.gov.au/sites/default/files/cmec-minutes-64.pdf
- Uzbay T, Goktalay G, Kayir H, Eker SS, Sarandol A, Oral S, Buyukuysal L, Ulusoy G, Kirli S. Increased plasma agmatine levels in patients with schizophrenia. Journal of Psychiatric Research 2013;47:1054-1060.
- Vahedi K, Domingo V, Amarenco P, Bousser MG. Ischaemic stroke in a sportsman who consumed MaHuang extract and creatine monohydrate for body building. Journal of Neurology, Neurosurgery and Psychiatry 2000;68(1):112-113.
- Vandenberghe K, Goris M, Van Hecke P, Van Leemputte M, Vangerven L, Hespel P. Long-term creatine intake is beneficial to muscle performance during resistance training. Journal of Applied Physiology 1997;83(6):2055-2063.
- Vecchiet L, Di Lisa F, Pieralisi G, Ripari P, Menabo R, Giamberardino MA, Siliprandi N. Influence of L-carnitine administration on maximal physical exercise. European Journal of Applied Physiology 1990;61:486-490.
- Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. American Journal of Clinical Nutrition 2009;89(5):1468-1475.
- Viribay A, Fernández-Landa J, Castañeda-Babarro A, Collado PS, Fernández-Lázaro D, Mielgo-Ayuso J. Effects of citrulline supplementation on different aerobic exercise performance outcomes: a systematic review and meta-analysis. Nutrients. 2022;14(17):3479.
- Volek JS, Duncan ND, Mazzeti SA, Staron RS, Putukian M, Gomez AL, Pearson DR, Fink WJ, Kraemer WJ. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Medicine and Science in Sports Exercise 1999;31(8):1147-1156.
- Volek JS, Kraemer WJ, Rubin MR, Gomez AL, Ratamess NA, Gaynor P. L-carnitine L-tartrate supplementation favorably affects markers of recovery from exercise stress. American Journal of Physiology-Endocrinology and Metabolism 2002;282:E474-E482.
- Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Buono MD, Lee KS, Leiter LA, Nam KY, Arnason JT, Choi M, Naeem A. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutrition, Metabolism & Cardiovascular Diseases 2008;18(1):46-56.
- Wall BT, Stephens FB, Constantin-Teodosiu D, Marimuthu K, Macdonald IA, Greenhaff PL. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. The Journal of Physiology 2011;589(4):963-973.
- Waugh WH, Daeschner III CW, Files BA, McConnell ME, Strandjord SE Oral Citrulline as arginine precursor may be beneficial in sickle cell disease: early phase two results. Journal of the National Medical Association 2001;93(10):362-371.
- WHO 1999: World Health Organization (WHO) Monographs on Selected Medicinal Plants: Volume 1. Geneva (CHE): World Health Organization.
- Wong A, Alvarez-Alvarado S, Jaime SJ, Kinsey AW, Spicer MT, Madzima TA, Figueroa A. Combined whole-body vibration training and L-citrulline supplementation improves pressure wave reflection in obese postmenopausal women. Applied Physiology and Nutrition Metabolism. Applied Physiology, Nutrition, and Metabolism 2016;41:292-297.
- Wutzke KD, Lorenz H. The effect of L-carnitine on fat oxidation, protein turnover; and body composition in slightly overweight subjects. Metabolism 2004;53(8):1002-1006.
- Xie S, Li S, Shaharudin S. The effects of combined exercise with citrulline supplementation on body composition and lower limb function of overweight older adults: A systematic review and meta-analysis. Journal of sports science & medicine. 2023 Sep;22(3):541.
- Zimmerman DR. Zimmerman's Complete Guide to Nonprescription Drugs, 2nd edition. Detroit (MI): Gale Research Inc.; 1992.
References Reviewed
- Baer DJ, Stote KS, Paul DR, Harris GK, Rumpler WV, Clevidence BA. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. The Journal of Nutrition 2011;141(8):1489-1494.
- Cockburn E, Stevenson E, Hayes PR, Robson-Ansley P, Howatson G. Effect of milk-based carbohydrate-protein supplement on the attenuation of exercise-induced muscle damage. Applied Physiology, Nutrition and Metabolism 2010; 35(3):270-277.
- European Food Safety Authority. Scientific Opinion on the substantiation of health claims related to caffeine and increase in physical performance during short-term high-intensity exercise, increase in endurance performance, increase in endurance capacity and reduction in the rated perceived exertion/effort during exercise pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2011;9(4):2053.
- Ivy JL, Katz AL, Cutler CL, Sherman WM, Coyle EF. Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. Journal of Applied Physiology 1988;64(4):1480-1485.
- Millard-Stafford M, Warren GL, Thomas LM, Doyle JA, Snow T, Hitchcock K. Recovery from run training: efficacy of a carbohydrate-protein beverage? International Journal of Sport Nutrition and Exercise Metabolism 2005;15(6):610-624.
- Saunders MJ, Kand MD, Todd MK. Effects of a Carbohydrate-Protein Beverage on Cycling Endurance and Muscle Damage. Physical Fitness and Performance 2004;36(7):1233-1328.
- Suzuki A, Charlton MR, Lymp JF, Jorgensen RA, Keach J, Petz J, Angulo P, Lindor K. A Pilot Study: No therapeutic effect of L-Alanine in patients with Nonalcoholic steatohepatitis. Food and Nutrition Sciences 2010;1(2):67-73.
- Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J. International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB). Journal of the International Society of Sports Nutrition 2013; 10:6.