THUJA - THUJA OCCIDENTALIS - Topical
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization. It is not intended to be a comprehensive review of the medicinal ingredient.
Notes
- Text in parentheses is additional optional information which can be included on the label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or statements are synonymous. Either term or statement may be selected by the applicant on the label.
Date
February 28, 2025
Proper name(s), Common name(s), Source information
| Proper name(s) | Common name(s) | Source information | ||
|---|---|---|---|---|
| Source material(s) | Part(s) | Preparation(s) | ||
| Thuja occidentalis |
|
Thuja occidentalis | Herb top | Dry |
References: Proper name: USDA 2024; Common names: Gardner and McGuffin 2013; Source information: BHP 1983; Felter and Lloyd 1983.
Route of Administration
Topical
Dosage Form(s)
Acceptable dosage forms when used according to the requirements indicated in this monograph: Cream; Gel; Liquid; Lotion; Ointment; Paste; Salve; Solution; Topical liquid (Bartram 1998; Williamson 1988; Wren 1988).
Use(s) or Purpose(s)
- (Traditionally) used in Herbal Medicine to help remove warts on the hands and feet (Hoffmann 2003; Williamson 2003; BHP 1983; Felter and Lloyd 1983).
- Used in Herbal Medicine to help relieve fungal infections such as ringworm (Hoffmann 2003; Williamson 2003).
Notes
For multi-ingredient products:
- To prevent the product from being represented as a “traditional medicine”, any indicated traditional claim must refer to the specific medicinal ingredient(s) and recognized traditional system of medicine from which the claim originates when 1) both traditional and modern claims are present or 2) when claims originate from multiple systems of traditional medicine (e.g., Thuja is traditionally used in Herbal Medicine to help remove warts on the hands and feet).
- When ALL of the medicinal ingredients (MIs) in the product are used within the SAME identified system of traditional medicine AND the product makes ONLY traditional claims, listing of MIs in the traditional claim(s) is not required.
Dose(s)
Subpopulation(s)
Adults 18 years and older
Quantity(ies)
Note: On the PLA form, quantities can be expressed as percentage weight by weight (% w/w), percentage weight by volume (% w/v) or percentage volume by volume (% v/v), depending on the product formulation. On the product label, quantities can also be expressed in other equivalent concentration units (e.g., mg/mL).
Methods of preparation: Non-Standardized Liquid Extracts (Decoction, Decoction concentrate, Fluid extract, Infusion, Infusion concentrate, Tincture)
10 - 100% dried herb top extract preparation in the finished product (Bartram 1998; Williamson 1988; Wren 1988; BHP 1983).
Note: The extract ratio must be between 1:1 and 1:10. The formulation must be prepared in a way which is equivalent to a quantity of 100 to 200 milligrams crude dried herb tops for 1 gram of finished product. For example, for a tincture prepared with a 1:5 w/v ratio, the concentration of tincture in the finished product must be at least 50% (100 - 200 mg crude dried herb tops * 5 w/v (dilution) = 500 mL liquid extract in 1 mL finished product = 50% v/v extract preparation in the finished product).
Methods of preparation: Non-Standardized Dry Extracts (Extract dry)
0.5 - 10% dried herb top extract preparation in the finished product (Bartram 1998; Williamson 1988; Wren 1988; BHP 1983).
Notes:
- The extract ratio must be between 2:1 and 20:1 for dry extracts. The formulation must be prepared in a way which is equivalent to a quantity of 100 to 200 milligrams crude dried herb tops for 1 gram of finished product. For example, for a 4:1 w/w dry extract, the concentration of dry extract in the finished product must be between 2.5 to 5% (100 - 200 mg crude dried herb tops / 4 w/w (concentration) = 25 - 50 mg dry extract in 1 g finished product = 2.5 - 5% w/w extract preparation in the finished product).
- Solvents allowed for this method of preparation as part of this monograph are ethanol and/or water only.
Direction(s) for use
Products used to help remove warts
- Wash affected area and dry thoroughly.
- Apply 1 drop at a time to cover each wart and let dry, up to 3 times daily.
Products used to help relieve fungal infections
- Wash affected area and dry thoroughly.
- Apply a thin layer over affected area, up to 3 times daily.
- Do not bandage.
Duration(s) of use
No statement required.
Risk Information
Caution(s) and warning(s)
- For external use only.
- Ask a health care practitioner/health care provider/health care professional/doctor/physician if symptoms persist or worsen.
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have diabetes or poor blood circulation (Berardi et al. 2002).
- Keep out of reach of children. If swallowed, call a poison control centre or get medical help right away.
Contraindication(s)
Do not use on the face or genital area. If contact occurs, rinse thoroughly with water (EMA 1999).
Known adverse reaction(s)
No statement required.
Non-medicinal ingredients
Must be chosen from the current Natural Health Product Ingredient Database (NHPID) and must meet the limitations outlined in the database.
Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations.
Specifications
- The finished product specifications must be established in accordance with the requirements described in the Natural and Non-prescription Health Products Directorate (NNHPD) Quality of Natural Health Products Guide.
- The medicinal ingredient must comply with the requirements outlined in the NHPID.
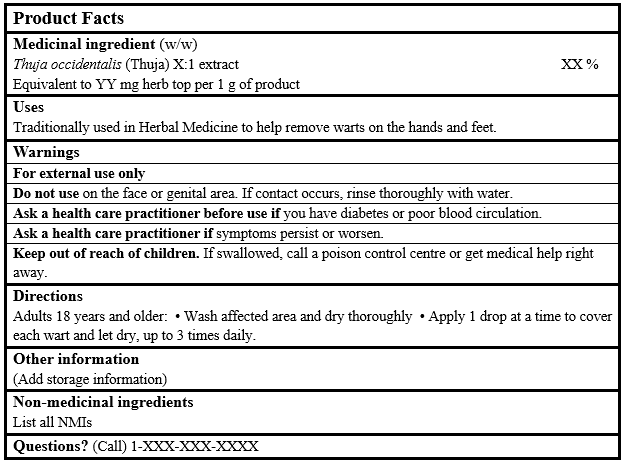
EXAMPLE OF PRODUCT FACTS:
Consult the Guidance Document, Labelling of Natural Health Products for more details.
References Cited
- Bartram T. Bartram's Encyclopedia of Herbal Medicine. New York (NY): Marlowe and Company; 1998.
- Berardi RR, DeSimone EM, Newton GD, Oszko MA, Popovich NG, Rollins CJ, Shimp LA, Tietze KJ, editors. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care, 13th edition. Washington (DC): American Pharmaceutical Association; 2002.
- BHP 1983: British Herbal Pharmacopoeia. Cowling (UK): British Herbal Medical Association; 1983.
- EMA: The European Agency for the Evaluation of medicinal Products. Committee for Veterinary Medicinal Products. Thuja occidentalis. London: EMA/MRL/602/99-FINAL; 1999.
- Felter HW, Lloyd JU. King's American Dispensatory, Volume 2, 18th edition. Sandy (OR): Eclectic Medical Publications; 1983 [Reprint of 1898 original].
- Gardner Z, McGuffin M, editors. American Herbal Products Association's Botanical Safety Handbook. 2nd Edition. Boca Raton (FL): Taylor and Francis Group; 2013.
- Hoffmann D. Medical Herbalism. Rochester (VT): Healing Arts Press; 2003.
- USDA 2024: United States Department of Agriculture Agricultural Research Service (USDA ARS), Germplasm Resources Information Network (GRIN) - Global. U.S. National Plant Germplasm System. [Accessed 2024 June 12]. Available from: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysearch
- Williamson EM, Evans FJ, Wren RC. Potter's New Cyclopaedia of Botanical Drugs and Preparations. Saffron Walden (UK): C.W. Daniel Company Limited; 1988.
- Williamson EM. Potter's Herbal Cyclopaedia: The Authoritative Reference work on Plants with a Known Medical Use. Saffron Walden (UK): The C.W. Daniel Company Limited; 2003.
- Wren RC. Potter's Cyclopedia of Botanical Drugs and Preparations. London (UK): Potter and Clark; 1907.
References Reviewed
- Burkhard PR, Burkhardt K, Haenggeli CA, Landis T. Plant-induced seizures: reappearance of an old problem. Journal of Neurology 1999;246(8):667-670.
- EC-SCF 2003: European Commission Scientific Committee on Food. Opinion of the Scientific Committee on Thujone. Brussels (B): European Commission Health and Consumer Protection Directorate-General. Scientific Committee on Food. Scientific Committee on Thujone; February 6, 2003, pp. 1-11. [Accessed 2024 June 12]. Available from: www.ec.europa.eu/food/fs/sc/scf/out162_en.pdf
- Stafstrom CE. Seizures in a 7-month-old child after exposure to the essential plant oil thuja. Pediatric Neurology 2007;37(6):446-448.