Nicotinamide Mononucleotide
This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization. It is not intended to be a comprehensive review of the medicinal ingredient.
Notes
- Text in parentheses is additional optional information which can be included on the label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or statements are synonymous. Either term or statement may be selected by the applicant on the label.
Date
August 28, 2024
Proper name(s), Common name(s), Source information
| Proper name(s) | Common name(s) | Source information |
|---|---|---|
| Source ingredient(s) | ||
| 3-(Aminocarbonyl)-1-(5-O-phosphonato-beta-D-ribofuranosyl)pyridinium |
|
Nicotinamide mononucleotide |
References: Proper name: NIH 2024; Common names: NIH 2024; Source information: NHPID 2024.
Route of administration
Oral
Dosage form(s)
This monograph excludes foods or food-like dosage forms as indicated in the Compendium of Monographs Guidance Document.
Acceptable dosage forms for oral use are indicated in the dosage form drop-down list of the web-based Product Licence Application form for Compendial applications.
Use(s) or Purpose(s)1
- Source of (an) antioxidant(s)/Provides (an) antioxidant(s) (Picciotto et al. 2016)2.
- Source of (an) antioxidant(s)/Provides (an) antioxidant(s) that help(s) fight/protect (cell) against/reduce (the oxidative effect of/the oxidative damage caused by/cell damage caused by) free radicals (Picciotto et al. 2016)2.
- A factor in the maintenance of good health (HC 2023).
- A factor in normal growth and development (HC 2023).
- Supports biological functions which play a key role in the maintenance of good health (HC 2023).
- Helps normal growth and development (HC 2023).
- Helps in energy metabolism/(and) tissue formation (HC 2023).
- Helps (to) maintain/support the body's ability to metabolize nutrients (HC 2023)3.
- NMN is a NAD+ precursor which is a factor in normal growth and development (Abdellatif et al. 2021; Blanco-Vaca et al. 2021; Covarrubias et al. 2021).
- NMN is a NAD+ precursor which helps to support biological functions which play a key role in the maintenance of good health (Abdellatif et al. 2021; Blanco-Vaca et al. 2021; Covarrubias et al. 2021).
- NMN is a NAD+ precursor which is an important cofactor in energy metabolism (Abdellatif et al. 2021; Blanco-Vaca et al. 2021; Covarrubias et al. 2021).
Notes:
- 1NMN is a vitamin B3 derivative and therefore uses associated with vitamin B3 as per the Multi-vitamin/mineral Supplements Monograph are acceptable.
- 2If NMN is combined with other medicinal ingredients with antioxidant properties, there is an option to use the antioxidant claims in plural. The singular should be used when the product only contains one chemical substance (e.g., NMN) as the medicinal ingredient associated with the antioxidant claim.
- 3This claim is not intended to convey that taking these vitamins helps to boost metabolism, upregulate a bodily system and/or directly convert food to energy. Inferring such claims would be misleading and is not permitted. In order to avoid any misinterpretation of this claim, the terms 'carbohydrates, fats, proteins, etc.' must not be used to further specify the term 'nutrients'.
- The above uses can be combined on the product label (e.g., Source of an antioxidant and helps normal growth and development).
Dose(s)
Subpopulation(s)
Adults 18 years and older
Quantity(ies)
Antioxidant
Not to exceed 1,200 milligrams of NMN, per day; and 600 milligrams per single dose (Huang 2022; Okabe et al. 2022; Liao et al. 2021).
Other uses
3 - 1,200 milligrams of NMN, per day; not to exceed 600 milligrams per single dose (HC 2023; Huang 2022; Okabe et al. 2022; Liao et al. 2021).
Direction(s) for use
No statement required.
Duration(s) of use
Products providing 250 milligrams or less of NMN, per day
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 12 weeks (Katayoshi et al. 2023; Okabe et al. 2022).
Products providing more than 250 milligrams of NMN, per day
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 8 weeks (Yi et al. 2023; Liao et al. 2021).
Risk information
Caution(s) and Warning(s)
- Ask a healthcare practitioner/health care provider/health care professional/doctor/physician before use if you are pregnant or breastfeeding.
- Ask a healthcare practitioner/health care provider/health care professional/doctor/physician before use if you have diabetes (Yoshino et al. 2021).
Contraindication(s)
No statement required.
Known Adverse Reaction(s)
No statement required.
Non-medicinal ingredients
Must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in the database.
Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations.
Specifications
- The finished product specifications must be established in accordance with the requirements described in the Natural and Non-prescription Health Products Directorate (NNHPD) Quality of Natural Health Products Guide.
- The medicinal ingredient must comply with the requirements outlined in the NHPID.
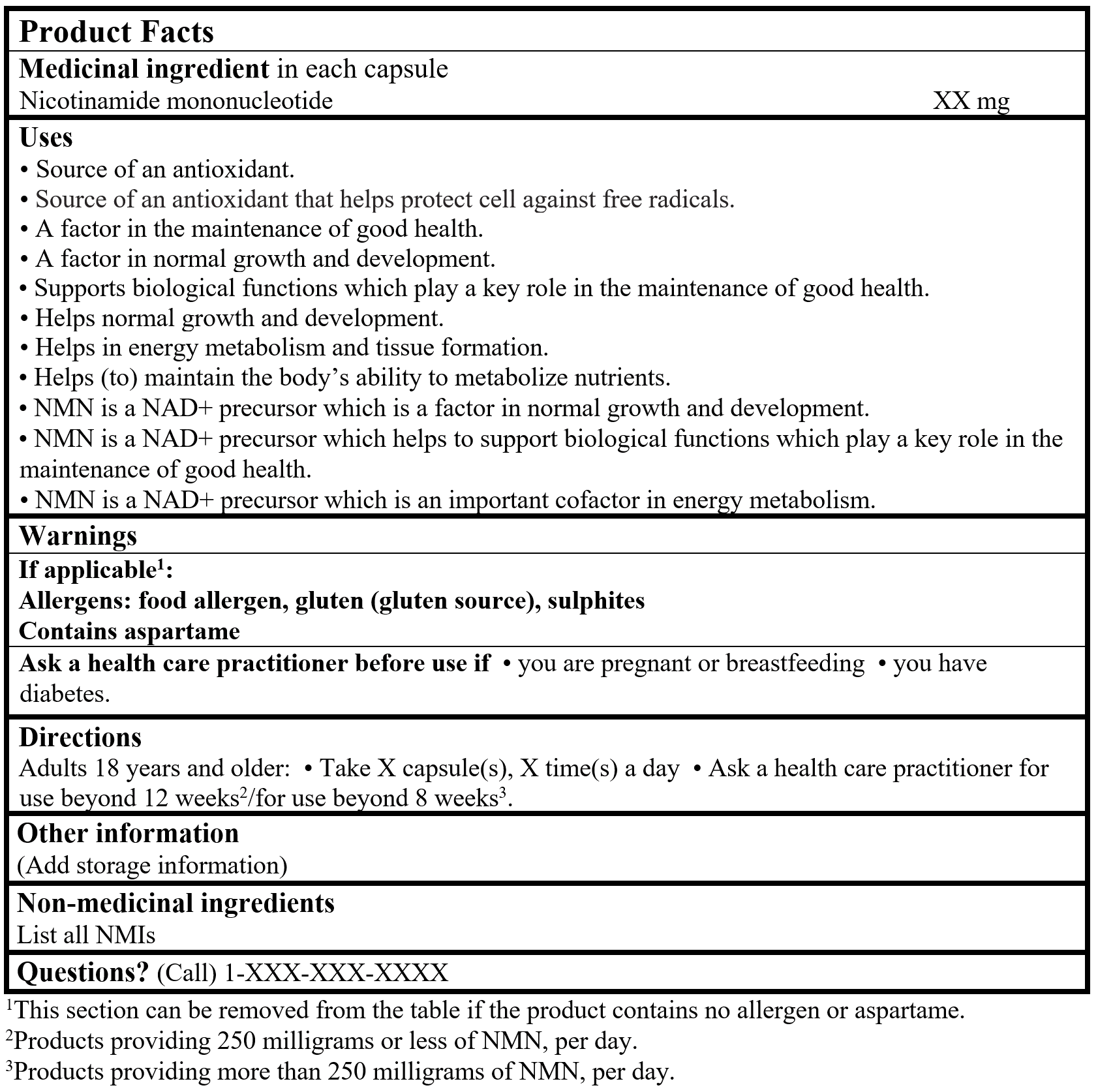
Example of product facts
Consult the Guidance Document, Labelling of Natural Health Products for more details.
References cited
- Abdellatif M, Sedej S, Kromer G. NAD+ Metabolism in Cardiac Health, Aging, and Disease. Circulation 2021;144(22):1795-1817.
- Blanco-Vaca F, Rotllan N, Canyelles M, Mauricio D, Escolà-Gil JC, Julve J. NAD+-Increasing Strategies to Improve Cardiometabolic Health? Frontiers in Endocrinology 2021;12.
- Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nature Reviews Molecular Cell Biology 2021;22:119-141.
- HC 2023: Health Canada. Multi-Vitamin/Mineral Supplements Monograph. [Accessed 2024 February 26]. Available from: https://webprod.hc-sc.gc.ca/nhpid-bdipsn/atReq.do?atid=multi_vitmin_suppl&lang=eng
- Huang H. A Multicentre, Randomised, Double Blind, Parallel Design, Placebo Controlled Study to Evaluate the Efficacy and Safety of Uthever (NMN Supplement), an Orally Administered Supplementation in Middle Aged and Older Adults. Front Aging 2022;3:851698.
- Katayoshi T, Uehata S, Nakashima N, Nakajo T, Kitajima N, Kageyama M, Tsuji-Naito K. Nicotinamide adenine dinucleotide metabolism and arterial stiffness after long-term nicotinamide mononucleotide supplementation: a randomized, double-blind, placebo-controlled trial. Sci Rep. 2023;13(1):2786.
- Liao B, Zhao Y, Wang D, Zhang X, Hao X, Hu M. Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind study. Journal of the International Society of Sports Nutrition 2021;18(1):1-9.
- NHPID 2024: Health Canada. Natural Health Products Ingredients Database. [Accessed 2024 February 26]. Available from: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do
- NIH 2024: National Institutes of Health. PubChem. Bethesda (MD): National Library of Medicine, US Department of Health & Human Services. [Accessed 2024 January 4]. Available from: https://pubchem.ncbi.nlm.nih.gov/
- Okabe K, Yaku K, Uchida Y, Fukamizu Y, Sato T, Sakurai T, Tobe K, Nakagawa T. Oral Administration of Nicotinamide Mononucleotide Is Safe and Efficiently Increases Blood Nicotinamide Adenine Dinucleotide Levels in Healthy Subjects. Front Nutr. 2022;9:868640.
- Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S-I, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging cell. 2016;15(3):522-530.
- Yi L, Maier AB, Tao R, Lin Z, Vaidya A, Pendse S, Thasma S, Andhalkar N, Avhad G, Kumbhar V. The efficacy and safety of β-nicotinamide mononucleotide (NMN) supplementation in healthy middle-aged adults: a randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial. Geroscience 2023;45(1):29-43.
- Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, ... Klein S. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 2021;372(6547);1224-1229.
References reviewed
- Igarashi M, Nakagawa-Nagahama Y, Miura M, Kashiwabara K, Yaku K, Sawada M, Sekine R, Fukamizu Y, Sato T, Sakurai T, Sato J, Ino K, Kubota N, Nakagawa T, Kadowaki T, Yamauchi T. Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men. NPJ Aging 2022;8(1);5.