KUTKI - PICRORHIZA KURROOA
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization. It is not intended to be a comprehensive review of the medicinal ingredient.
Notes
- Text in parentheses is additional optional information which can be included on the PLA and product label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or statements are synonymous. Either term or statement may be selected by the applicant.
Date
July 26, 2024
Proper name(s), Common name(s), Source information
| Proper name(s) | Common name(s) | Source information | ||
|---|---|---|---|---|
| Source material(s) | Part(s) | Preparation(s) | ||
| Picrorhiza kurrooa |
|
Picrorhiza kurrooa |
|
Dry |
References: Proper name: USDA 2024; Common names: USDA 2024; API 2001; Source information: WHO 2009; Duke et al. 2002; Williamson 2002.
Route of Administration
Oral
Dosage Form(s)
This monograph excludes foods or food-like dosage forms as indicated in the Compendium of Monographs Guidance Document.
Acceptable dosage forms for oral use are indicated in the dosage form drop-down list of the web-based Product Licence Application form for Compendial applications.
Use(s) or Purpose(s)
- Traditionally used in Ayurveda as a bitter tonic to help stimulate appetite and aid digestion (stomachic) (Williamson 2002; API 2001; Kapoor 2001).
- Traditionally used in Ayurveda as a hepatoprotectant/liver protectant (Williamson 2002; Kapoor 2001).
- Traditionally used in Ayurveda as a laxative for the relief of occasional constipation (Sudarshan 2005; API 2001; Kapoor 2001).
Notes
- The laxative claim is based on the Ayurvedic Medicine; however, the mode of action is unclear but may be comparable to stimulant laxatives.
- The above uses can be combined on the product label if from the same traditional or non-traditional system of medicine (e.g. Traditionally used in Ayurveda as a bitter tonic to help stimulate appetite and aid digestion and as a laxative for the relief of occasional constipation).
- For multi-ingredient products:
- To prevent the product from being represented as a "traditional medicine", any indicated traditional use claim must refer to the specific medicinal ingredient(s) and recognized traditional system of medicine from which the claim originates when 1) both traditional and modern claims are present or 2) when claims originate from multiple systems of traditional medicine (e.g. Kutki is traditionally used in Ayurveda as a bitter tonic to help stimulate appetite and aid digestion).
- When ALL of the medicinal ingredients (MIs) in the product are used within the SAME identified system of traditional medicine AND the product makes ONLY traditional claims, listing of MIs in the traditional claim(s) is not required
Dose(s)
Subpopulation(s)
Adults 18 years and older
Quantity(ies)
Methods of preparation: Dry, Powdered, Non-Standardized Ethanolic Extracts (Dry extract, Tincture, Fluid extract)
Bitter tonic; Liver protectant
1 - 3 grams of dried rhizome/root, per day (WHO 2009; API 2001)
Laxative
1.5 - 3 grams of dried rhizome/root, per day (Paranjape 2005)
Direction(s) for use
All products
Take a few hours before or after taking other medications or health products (HC 2009).
Duration(s) of Use
Laxative
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 7 days (HC 2009; Pray 2006; CPhA 2002).
Risk Information
Caution(s) and warning(s)
Bitter tonic; Laxative
Ask a health care practitioner/health care provider/health care professional/doctor/physician if symptoms persist or worsen.
Contraindication(s)
All products
- Do not use if you are pregnant or breastfeeding (Brinker 2010).
- Do not use if you have fever or any undiagnosed gastrointestinal trouble (HC 2009).
Known adverse reaction(s)
Bitter tonic; Liver protectant
When using this product you may experience a laxative effect (Sudarshan 2005; API 2001; Kapoor 2001).
Non-medicinal ingredients
Must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in the database.
Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations.
Specifications
- The finished product specifications must be established in accordance with the requirements described in the Natural and Non-prescription Health Products Directorate (NNHPD) Quality of Natural Health Products Guide.
- The medicinal ingredient must comply with the requirements outlined in the NHPID.
- Picrorhiza kurrooa is listed in Appendix II of the Convention on International Trade of Endangered Species of Wild Fauna and Flora (CITES). This species is protected under Canada's Wild Animal and Plant Protection and Regulation of International and Interprovincial Trade Act (WAPPRIITA) and its Regulations. Please ensure the required CITES import/export permit accompanies each shipment. For more information, see https://www.canada.ca/en/environment-climate-change/services/convention-international-trade-endangered-species/permits.html.
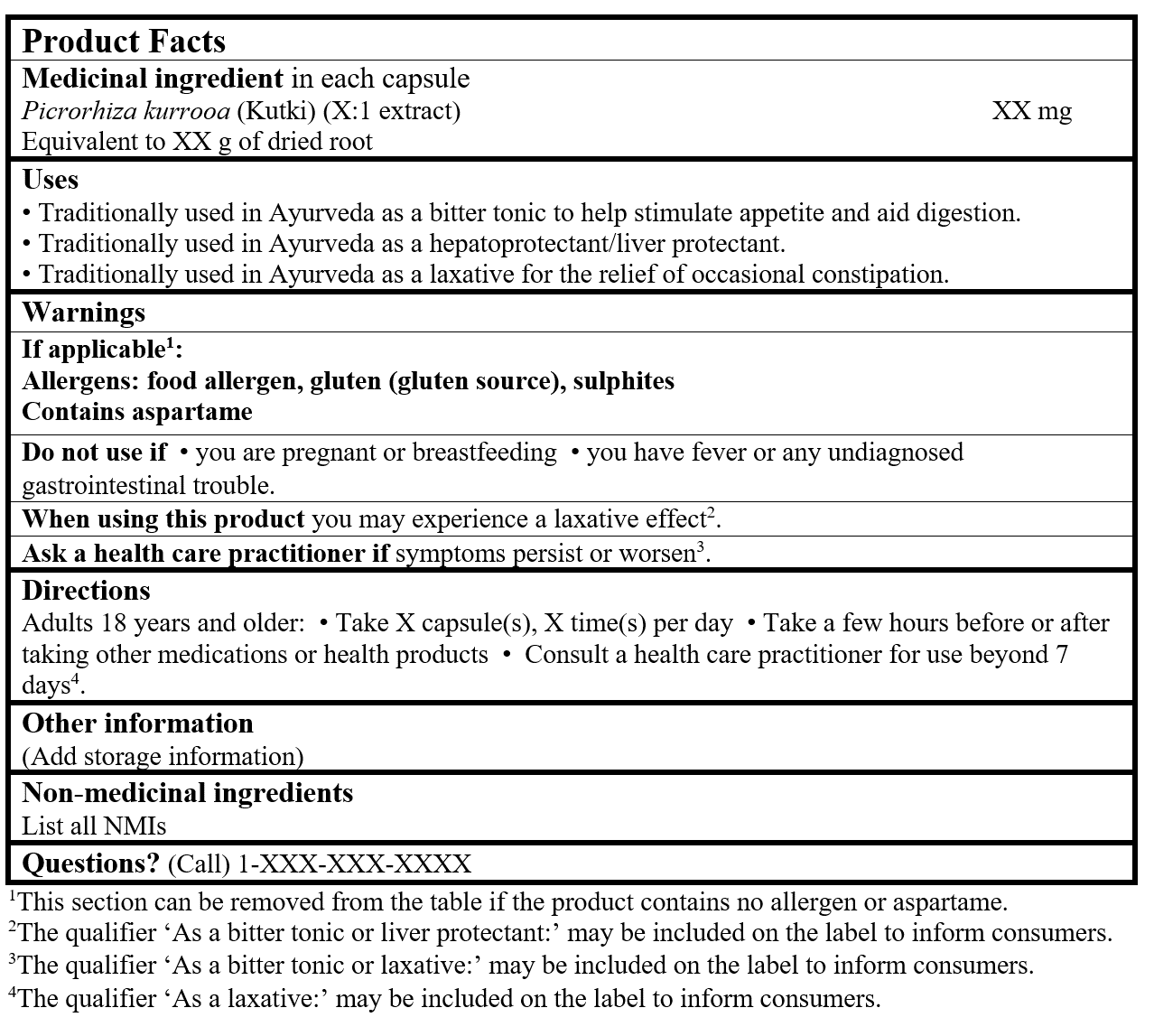
EXAMPLE OF PRODUCT FACTS:
Consult the Guidance Document, Labelling of Natural Health Products for more details.
References Cited
- API 2001: The Ayurvedic Pharmacopoeia of India. Part I, Volume II, 1st edition. Delhi (IN): The Controller of Publications; 1991. [Accessed 2018 August 14]. Available from: http://www.ayurveda.hu/api/API-Vol-2.pdf
- Brinker F. Herbal Contraindications and Drug Interactions: Plus Herbal Adjuncts with Medicines, expanded 4th edition. Sandy (OR): Eclectic Medical Publications; 2010.
- CPhA 2002: Repchinsky C, editor-in-chief. Patient Self-Care: Helping Patients Make Therapeutic Choices. 1st edition. Ottawa (ON): Canadian Pharmacists Association; 2002.
- Duke JA, Bogenschutz-Godwin MJ, duCellier J, Duke PK. Handbook of Medicinal Herbs.2nd edition. Boca Raton (FL): CRC Press; 2002.
- HC 2009: Laxatives: General - Laxatives - Labelling Standard. Ottawa (ON): Drugs and Health Products; Health Canada. [Accessed 2024 January 6]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/nonprescription-drugs-labelling-standards/laxatives-labelling-standards-non-prescription-drugs.html
- Kapoor LD. Handbook of Ayurvedic Medicinal Plants. Baton Roca (FL): CRC Press LLC; 2001.
- Paranjpe P. Indian Medicinal Plants: Forgotten Healers: A Guide to Ayurvedic Herbal Medicine. Delhi (IN): Chaukhamba Sanskrit Pratishthan; 2005.
- Pray WS. Non-Prescription Product Therapeutics, 2nd edition. New York (NY): Lippincott Williams & Wilkins; 2006.
- Sudarshan SR. Encyclopaedia of Indian Medicine, Materia Medica-Herbal Drugs.Volume 4.Banglore (IN): Popular Prakashan; 2005.
- USDA 2024: United States Department of Agriculture, Agricultural Research Service, National Genetic Resources Program. Germplasm Resources Information Network (GRIN) - Global. U.S. National Plant Germplasm System. [Accessed 2024 January 6]. Available from: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysearch
- WHO 2009: World Health Organization. WHO Monographs on Selected Medicinal Plants, Volume 4. Geneva (CH): World Health Organization; 2009.
- Williamson EM, editor. Major Herbs of Ayurveda. Edinburgh (GB): Churchill Livingstone; 2002.
References Reviewed
- Ansari RA, Tripathi SC, Patnaik GK, Dhawan BN. Antihepatotoxic properties of picroliv: an active fraction from rhizomes of Picrorhiza kurrooa. Journal of Ethnopharmacology 1991;34: 61-68.
- Lupper S. A Review of Plants Used in the Treatment of Liver Disease: Part 1. Alternative Medicine Review 1999;3(6):410-421.
- Nadkarni AK. Dr. KM Nadkarni's Indian Materia Medica, With Ayurvedic, Unani-Tibbi, Siddha, Allopathic, Homeopathic, Naturopathic & Home Remedies, Appendices & Indexes, Volume One. Bombay Popular Prakashan;1976.
- Saraswat B, Visen PKS, Parnaik GK, Dhawan BN. Ex vivo and in vivo investigations of picroliv from Picrorhiza kurroa in an alcohol intoxication model in rats. Journal of Ethnopharmacology 1999;66:263-269.
- Shetty SN, Mengi S, Vaidya R, Vaidya ADB. A study of standardized extracts of Picrorhiza kurroa Royle ex Benth in experimental nonalcoholic fatty liver disease. Journal of Ayurveda Integrative Medicine 2010;1(3):2013-2010.
- Shukla B, Visen PKS, Patnaik GK, Dhawan BN. Choleretic Effect of Picroliv, the Hepatoprotective Principle of Picrorhiza kurroa. Planta Medica 1991;57:29-33.
- Vaidya AB, Antarkar DS, Doshi JC, Bhatt AD, Ramesh VV, Vora PV, Perissond DD, Baxi AJ, Kale PM. Picrohiza kurroa (Kutaki) Boyle ex Benth as a hepatoprotective agent - experimental & clinical studies. Journal of Postgraduate Medicine 1996;42(4):105-108.