Athlete's Foot Treatments
Date
2024-09-27
Foreword
This monograph is intended to replace the existing Athlete's Foot Treatments Monograph of December 07, 2018. This monograph describes the requirements necessary to receive market authorization [i.e. a Drug Identification Number (DIN) or a Natural Product Number (NPN)], for athlete's foot treatment products. The monograph identifies the permitted medicinal and non-medicinal ingredients, concentrations, indications, directions and conditions of use for these products to be market authorized without the submission to Health Canada of additional evidence. Products which do not meet the criteria outlined in this document can apply for market authorization outside of the monograph stream.
Applicants are reminded that athlete's foot treatments products, like other non-prescription drugs or natural health products, are subject to the Food and Drug Regulations or the Natural Health Products Regulations administered by the Natural and Non-prescription Health Products Directorate (NNHPD). This includes requirements related to labelling, manufacturing and product specifications. Additional information on labels, outside of those specified in the monograph, such as additional directions for use and/or non-therapeutic claims are acceptable as long as they meet the Guidelines for the Nonprescription and Cosmetic Industry Regarding Non-therapeutic Advertising and Labelling Claims, the Guidelines for Consumer Advertising of Health products for Nonprescription drugs, Natural Health Products, Vaccines and Medical Devices, and are not false, misleading or counterintuitive to the use of the product.
Notes:
- Text in parentheses is additional optional information which can be included on the label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or the statements are synonymous. Either term or statement may be selected by the applicant on the label.
Medicinal Ingredients
Athlete's foot treatment products are classified as natural health products (NHPs) if they contain only one or several ingredients from Table 1. Applicants applying for an NPN can access the appropriate forms and guidance at: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription.html.
Athlete's foot treatment products are classified as non-prescription drugs (NPDs) if they contain an ingredient from Table 2. Applicants applying for a DIN can access the appropriate forms and guidance at: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents.html.
Proper name(s), Common name(s), Source information
| Proper name(s)1 | Common name(s)1 | Source information1 | Quantity |
|---|---|---|---|
| Source ingredient(s) | |||
| 10-Undecenoic acid |
|
Undecenoic acid | 10-25% |
| 10-Undecenoic acid, calcium(2+) salt | Calcium undecylenate | Calcium undecylenate | 10-25% |
| 10-Undecenoic acid, copper(2+) salt | Copper undecylenate | Copper undecylenate | 10-25% |
| 10-Undecenoic acid, zinc(2+) salt | Zinc undecylenate | Zinc undecylenate | 10-25% |
|
Povidone-iodine | Povidone-iodine | 10% |
1At least one of the following references was consulted per proper name, common name, and source information: NHPID 2024; RSC 2023; US FDA 2021; Sweetman 2017.
2Quantities are expressed as percentage weight by volume (% w/v). The following reference was consulted for the quantity: US FDA 2021.
| Proper name(s) | Common name(s) | Quantity |
|---|---|---|
|
Chlorphenesin | 0.5-1% |
| 5-Chloro-7-iodo-8-quinolinol |
|
3% |
|
Haloprogin | 1% |
|
Tolnaftate | 1% |
Route of administration
Topical
Dosage form(s)
Acceptable dosage forms for NHPs
- Aerosol; Aerosol, metered dose; Aerosol, spray; Cream; Gel; Liquid; Lotion; Ointment; Paste; Powder; Solution; Spray; Spray, bag-on-valve; Spray, metered dose; Spray, suspension; Swab, medicated; Topical liquid; Wipe.
Acceptable dosage forms for NPDs
- Aerosol; Aerosol Foam; Cream; Gel; Lotion; Ointment; Paste; Powder; Solution; Spray; Spray, bag-on-valve; Spray, metered dose; Swab; Wipe.
Use(s) or Purpose(s)
All products (Self-Care Framework Category I)
- For the treatment of athlete's foot (US FDA 2021).
- Cures athlete's foot (US FDA 2021).
- Kills athlete's foot fungus (US FDA 2021).
- Relieves (itching, scaling, burning, cracking, redness, soreness, irritation of) athlete's foot (US FDA 2021).
Products containing tolnaftate
- With daily use, for the prevention of athlete's foot, for individuals with recurring problems (US FDA 2021).
Dose(s)
Subpopulation(s)
Children 2 to 11 years, Adolescents 12 to 17 years, Adults 18 years and older
Quantity(ies)
See Tables 1 and 2.
Permitted combinations
Two or more of the following ingredients from Table 1 may be combined, provided that the combined ingredients provide a total undecylenate concentration of 10 - 25%:
- undecylenic acid
- calcium undecylenate
- copper undecylenate
- zinc undecylenate
Direction(s) for use
All products except products with a claim for the prevention of athlete's foot only
- Clean skin with soap and water and dry thoroughly (US FDA 2021).
- Apply a thin layer over affected area morning and night for full treatment period of 4 weeks (US FDA 2021).
- Pay special attention to spaces between toes (US FDA 2021).
- Wear well fitting, ventilated shoes and cotton socks (US FDA 2021).
- Supervise children when they use this product (US FDA 2021).
Products containing tolnaftate with a claim for the prevention of athlete's foot
- Clean skin with soap and water and dry thoroughly (US FDA 2021).
- For prevention, apply a thin layer to the feet once or twice daily (US FDA 2021).
- Pay special attention to spaces between toes (US FDA 2021).
- Wear well fitting, ventilated shoes and cotton socks (US FDA 2021).
- Supervise children when they use this product (US FDA 2021).
Aerosols, sprays and powders
Avoid inhaling or exposing others to (the) spray/powder.
Duration(s) of use
No statement required
Risk information
Caution(s) and warning(s)
All products
- For external use only (US FDA 2021).
- When using this product avoid contact with eyes. If contact occurs, rinse thoroughly with water (US FDA 2021).
- Stop use and ask a doctor/physician/health care practitioner/health care provider/health care professional if symptoms worsen or last for more than 4 weeks (US FDA 2021).
- Keep out of reach of children. If swallowed, call a poison control centre or get medical help right away.
Products containing povidone-iodine
Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have a thyroid disease (US FDA 2013).
Contraindication(s)
All products
Do not use for infections of the nails (US FDA 2021).
Products containing povidone-iodine
Do not use if you are pregnant or breastfeeding (US FDA 2013).
Known adverse reaction(s)
Products containing povidone-iodine
Rare anaphylactic reactions have been known to occur (Gray et al. 2013; Palobart et al. 2009; Yoshida et al. 2008).
Non medicinal ingredients
Ingredients must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in that database, the Food and Drug Regulations, and the current Cosmetic Ingredient Hotlist, when relevant.
Storage conditions
- Must be established in accordance with the requirements described in the Natural Health Products Regulations or the Food and Drug Regulations.
- If the product is flammable or the if the container is under pressure, storage condition and warnings are required by the Consumer Chemicals and Containers Regulations.
Specifications
This monograph describes those requirements that are specific to this class of non-prescription drugs and to natural health products. Any change to the manufacturing process that impacts the safety and efficacy of the ingredients, such as the use of novel technology (e.g. nanotechnology), requires supporting data and will be reviewed outside the monograph
For products containing a Table 1 NHP medicinal ingredient
The finished product specifications must be established in accordance with the requirements described in the NNHPD Quality of Natural Health Products Guide. The medicinal ingredient must comply with the requirements outlined in the NHPID.
For products containing a Table 2 NPD medicinal ingredient
Requirements described in the Regulations to the Food and Drugs Act must be met.
For products containing a Table 1 NHP medicinal ingredient:
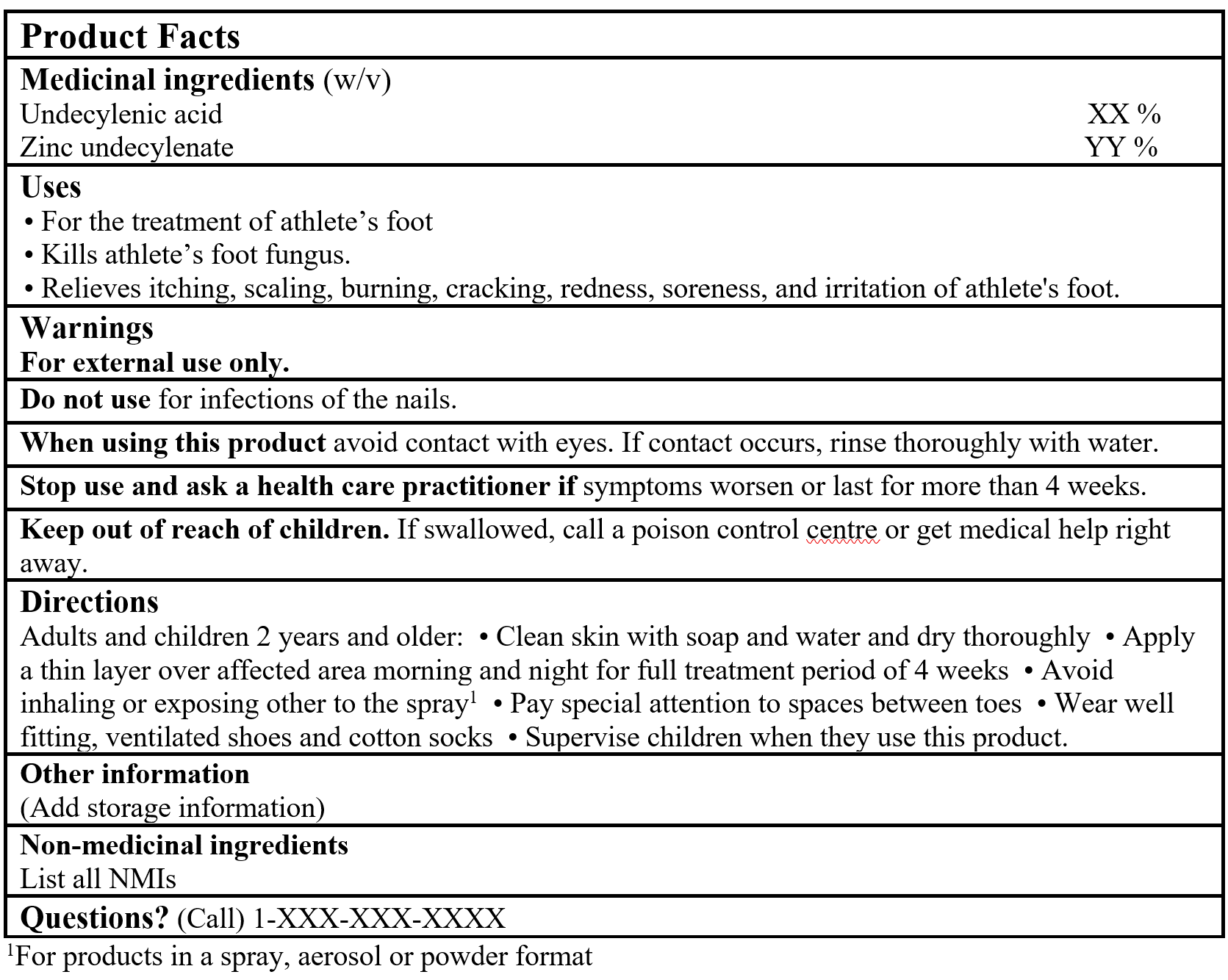
Example of product facts
Consult the Guidance Document, Labelling of Natural Health Products for more details.
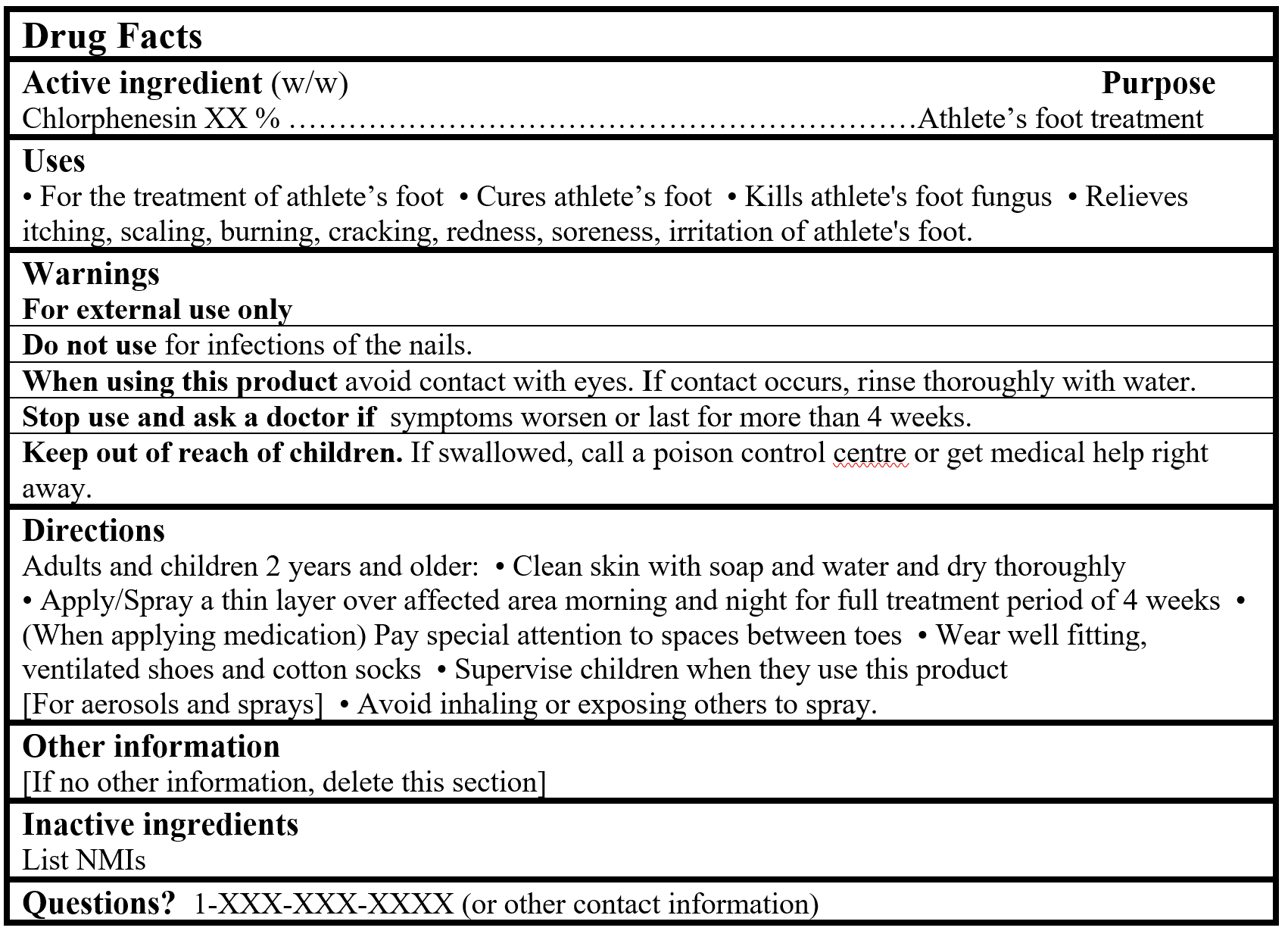
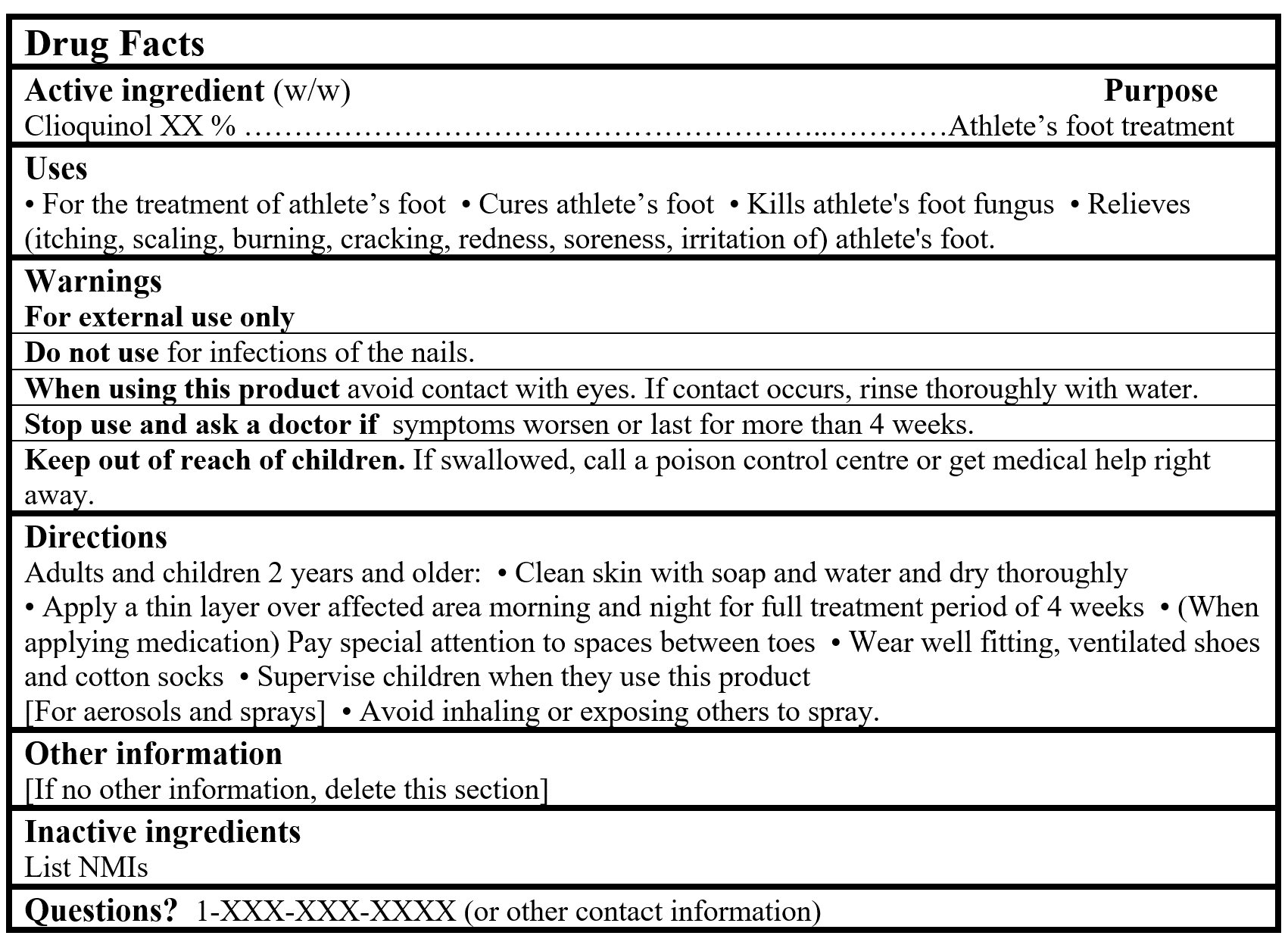
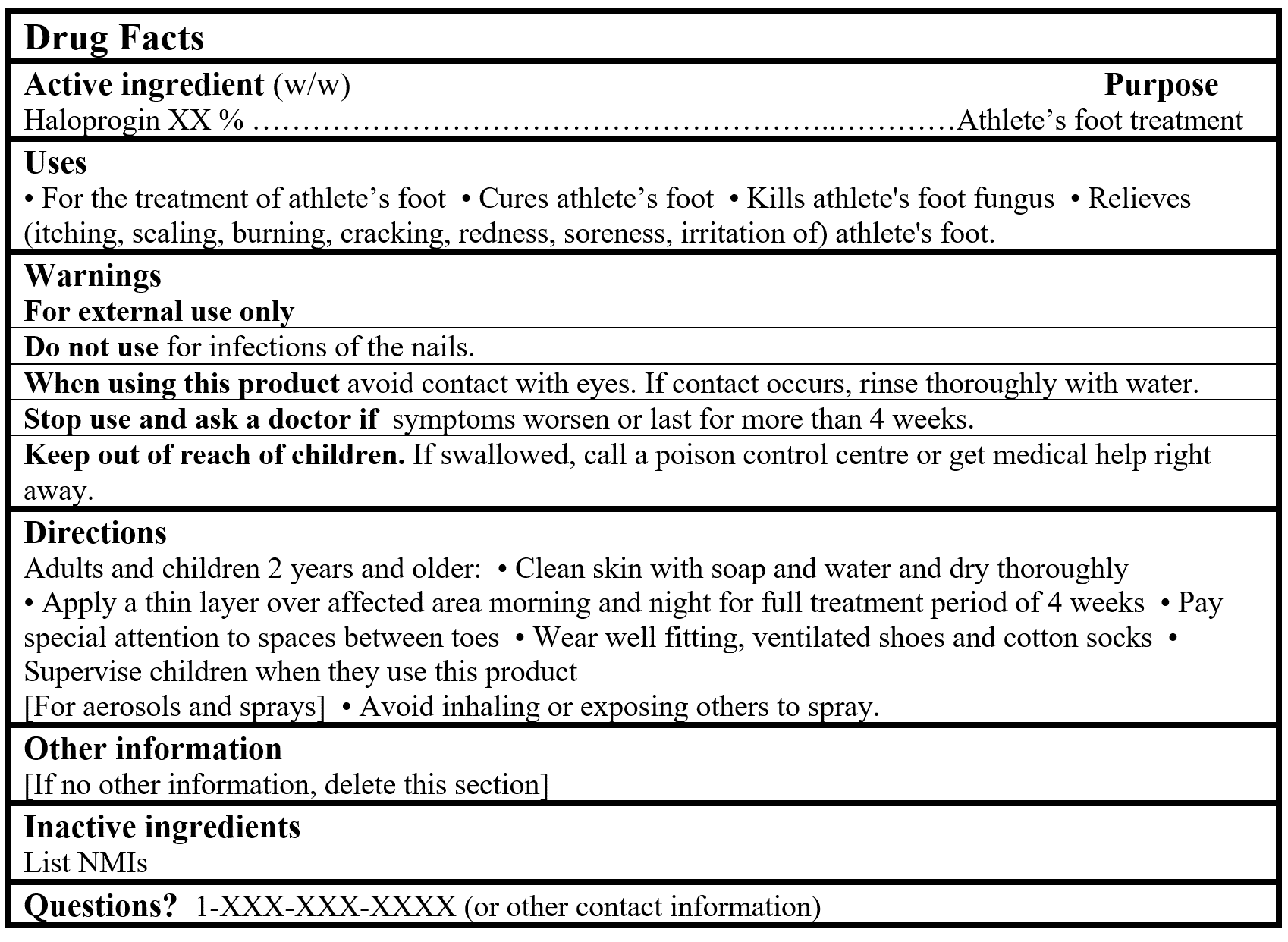
For products containing a Table 2 NPD medicinal ingredient:
Drug Facts Tables (Format Optional for Self-Care Category I)
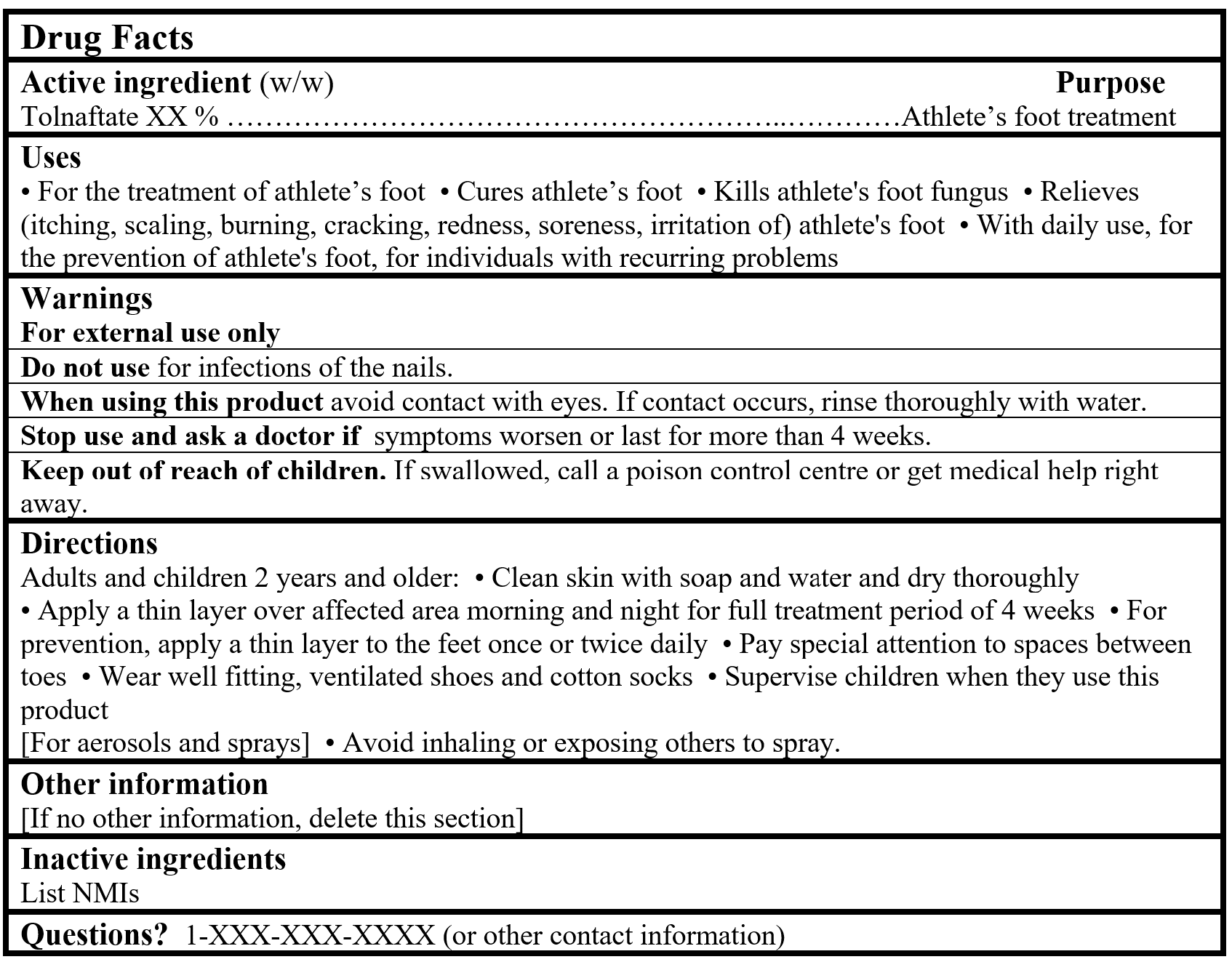
References
- Compendium of therapeutics for minor ailments (CTMA). Power, B, et al., editors. Canadian Pharmacists Association ; 2016.
- Compendium of products for minor ailments (CPMA). Power, B, et al., editors. Canadian Pharmacists Association ; 2016.
- Drug Facts and Comparisons, 2016. Facts and Comparisons Division, Wolters Kluwer; 2017 edition (Oct. 28 2016)
- Drugdex, Micromedex Inc. 2.0
- Goodman and Gilman's The Pharmacological Basis of Therapeutics, 13th Edition 2017 Brunton L., Knollmann B., Hilal-Dandan R. McGraw-Hill Education.
- Gray Pe, Katelaris CH, Lipson D. Recurrent anaphylaxis caused by topical povidone-iodine (Betadine). Journal of paediatrics and child health 2013;49(6):506-507.
- Krinsky DL, Ferreri SP, Hemstreet B, Hume AL, Newton GD, Rollins CJ, Tietze KJ. Handbook of Nonprescription Drugs: An interactive approach for Self-Care, 19th edition. Washington (DC): American Pharmaceutical Association; 2017.
- NHPID 2024. Natural Health Products Ingredients Database. [Accessed 2024 January 28]. Available from: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do
- Palobart C, Cros J, Orsel I, Nathan N. Anaphylactic shock to iodinated povidone. Annales françaises d'anesthésie et de réanimation 2009;28(2):168-170.
- Remington's Pharmaceutical Science, 13th Edition, 1996, Philadelphia College of Pharmaceutical Sciences
- RSC 2023: Royal Society of Chemistry. The Merck Index Online. [Accessed 2024 January 28]. Available from https://merckindex.rsc.org/.
- Sweetman SC, editor. Martindale: The Complete Drug Reference, 39th edition. Grayslake (IL): Pharmaceutical Press; 2017.
- US FDA 2013: United States Food and Drug Administration. 21 CFR Parts 310 and 333. Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use; Proposed Amendment of the Tentative Final Monograph; Reopening of Administrative Record. Federal Register, Volume 78, Number 242, December 17, 2013. [Accessed 2024 January 28]. Available from: https://www.federalregister.gov/documents/2013/12/17/2013-29814/safety-and-effectiveness-of-consumer-antiseptics-topical-antimicrobial-drug-products-for
- US FDA 2021: U.S. Food and Drug Administration. Over-the-Counter (OTC) Monograph M005: Topical Antifungal Drug Products for Over-the-Counter Human Use. Washington (DC): U.S. Food and Drug Administration, Department of Health and Human Services; 2021. [Accessed 2024 January 28]. Available from https://www.accessdata.fda.gov/drugsatfda_docs/omuf/OTC%20Monograph_M005-Topical%20Antifungal%20drug%20products%20for%20OTC%20Human%20Use%2012.16.2021.pdf
- Yoshida K, Sakurai Y, Kawahara S, Takeda T, Ishikawa T, Murakami T, Yoshioka A. Anaphylaxis to polyvinylpyrrolidone in povidone-iodine for impetigo contagiosum in a boy with atopic dermatitis. International archives of allergy and immunology 2008;146:169-173.