PLANT STANOL ESTERS
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization. It is not intended to be a comprehensive review of the medicinal ingredient.
Notes- Text in parentheses is additional optional information which can be included on the PLA and product label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or statements are synonymous. Either term or statement may be selected by the applicant.
Date
February 23, 2024
Proper name(s), Common name(s), Source information
| Proper name(s) | Common name(s) | Source information | |
|---|---|---|---|
| Source material(s) | Part(s) | ||
| Plant stanol esters | Plant stanol esters |
|
Seed |
|
Whole plant | ||
References: Proper name: FDA 2023, EC 2002, FDA 2001; Common name: FDA 2023, EC 2002, FDA 2001; Source information: USDA 2023, EC 2002, Kerckhoffs et al. 2002, FDA 2001.
Route of administration
Oral (FDA 2023; de Jong et al. 2008; Hallikainen et al. 2008; Goldberg et al. 2006; Chen et al. 2005; FDA 2001)
Dosage Form(s)
This monograph excludes foods or food-like dosage forms as indicated in the Compendium of Monographs Guidance Document.
Acceptable dosage forms for oral use are indicated in the dosage form drop-down list of the web-based Product Licence Application form for Compendial applications.
Use(s) or Purpose(s)
- Helps lower blood total and low density lipoprotein (LDL) cholesterol (FDA 2023; de Jong et al. 2008; Hallikainen et al. 2008; Goldberg et al. 2006; Chen et al. 2005; FDA 2001).
- Helps lower bad cholesterol (FDA 2023; de Jong et al. 2008; Hallikainen et al. 2008; Goldberg et al. 2006; Chen et al. 2005; FDA 2001).
- Helps maintain healthy cholesterol levels (FDA 2023; de Jong et al. 2008; Hallikainen et al. 2008; Goldberg et al. 2006; Chen et al. 2005; FDA 2001).
Note: The above uses can be combined on the product label (e.g., Helps lower blood total and low density lipoprotein (LDL) cholesterol and maintain healthy cholesterol level).
Dose(s)
Subpopulation(s)
Adults 18 years and older
Quantity(ies)
1.8 - 5.1 grams of Plant stanol esters per day, including at least 80 % of Combined Sitostanol and Campestanol, per day (FDA 2023; de Jong et al. 2008; Hallikainen et al. 2008; Goldberg et al. 2006; Chen et al. 2005; EC 2002; Kerckhoffs et al. 2002; FDA 2001).
Direction(s) for use
Take with food (FDA 2023; de Jong et al. 2008; Hallikainen et al. 2008; Goldberg et al. 2006; Chen et al. 2005; EC 2002; FDA 2001).
Duration(s) of use
No statement required.
Risk Information
Caution(s) and warning(s)
Ask a healthcare practitioner/health care provider/health care professional/doctor/physician before use if you are pregnant or breastfeeding.
Contraindication(s)
No statement required.
Known Adverse Reaction(s)
No statement required.
Non-medicinal Ingredients
Must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in the database.
Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations.
Specifications
- The finished product specifications must be established in accordance with the requirements described in the Natural and Non-prescription Health Products Directorate (NNHPD) Quality of Natural Health Products Guide.
- The medicinal ingredient must comply with the requirements outlined in the NHPID.
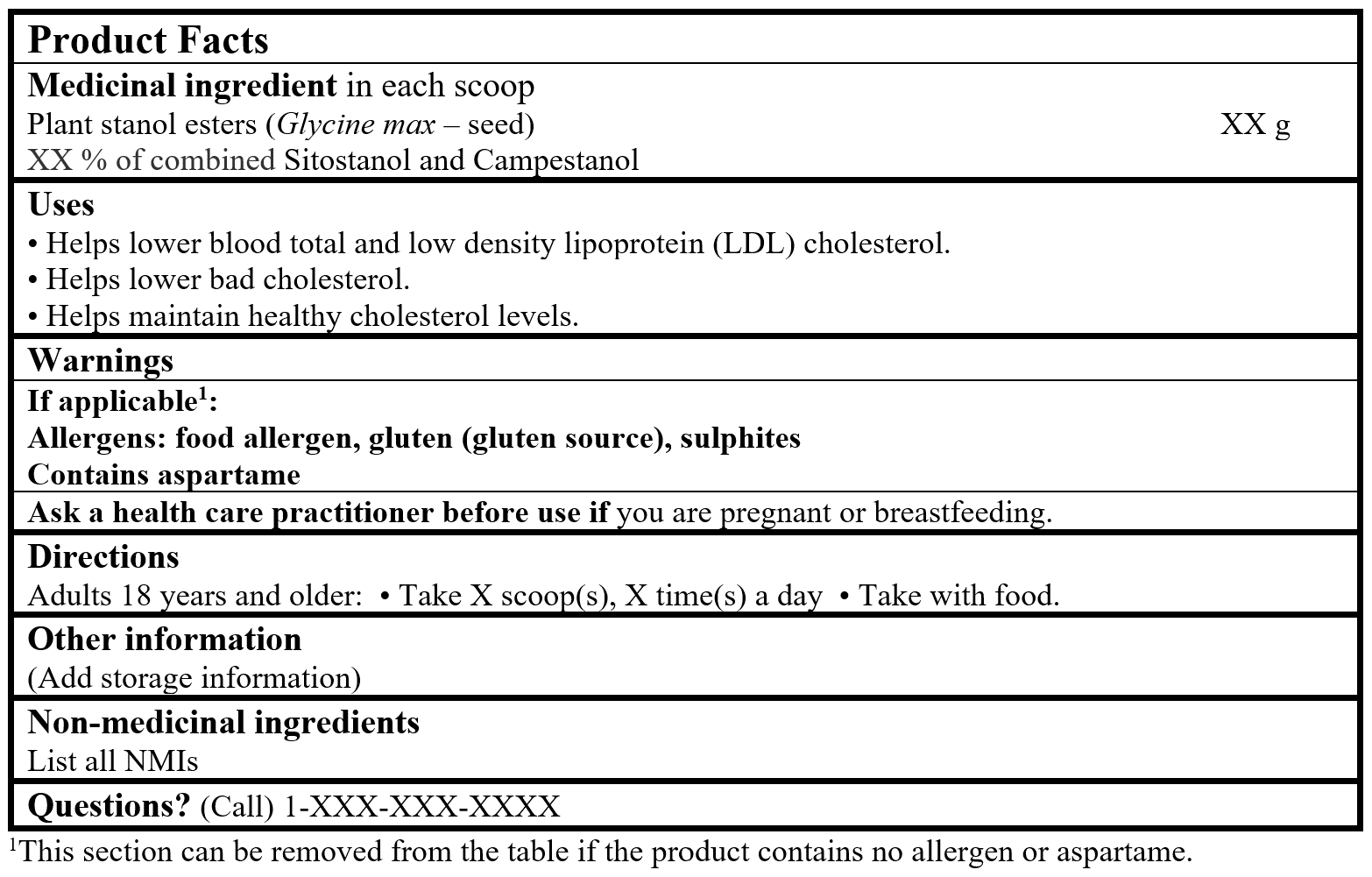
EXAMPLE OF PRODUCT FACTS:
Consult the Guidance Document, Labelling of Natural Health Products for more details.
References Cited
- Chen JT, Wesley R, Shamburek RD, Pucino F, Csako G. 2005. Meta-Analysis of Natural Therapies for Hyperlipidemia Plant Sterols and Stanols versus Policosanol for Hyperlipidemia. Pharmacotherapy. 25: 171-183.
- de Jong A, Plat J, Bast A, Godschalk RW, Basu S, Mensink RP. Eur J Clin Nutr. Effects of plant sterol and stanol ester consumption on lipid metabolism, antioxidant status and markers of oxidative stress, endothelial function and low-grade inflammation in patients on current statin treatment. 2008 Feb;62(2):263-273
- EC 2002: European Commission. General view of the Scientific Committee on Food on the long-term effects of the intake of elevated levels of phytosterols from multiple dietary sources, with particular attention to the effects on β-carotene. Health & Consumer Protection Directorate-General. [Accessed 2024 February 5]. Available from : https://food.ec.europa.eu/system/files/2020-12/sci-com_scf_out143_en.pdf
- FDA 2001: Food and Drug Administration, Department of Health and Human Services. Food labeling: health claims; plant sterol/stanol esters and coronary heart disease. Interim final rule; notice of extension of period for issuance of final rule. Federal Register. 66(109):30311-30313.
- FDA 2023: Food and Drug Administration. § 101.83 Health claims: plant sterol/stanol esters and risk of coronary heart disease (CHD). [Accessed 2024 February 1]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.83
- Goldberg AC, Ostlund RE Jr, Bateman JH, Schimmoeller L, McPherson TB, Spilburg CA. Effect of plant stanol tablets on low-density lipoprotein cholesterol lowering in patients on statin drugs. Am J Cardiol. 2006 Feb 1;97(3):376-379.
- Hallikainen M, Lyyra-Laitinen T, Laitinen T, Moilanen L, Miettinen TA, Gylling H. Effects of plant stanol esters on serum cholesterol concentrations, relative markers of cholesterol metabolism and endothelial function in type 1 diabetes. Atherosclerosis. 2008 Aug;199(2):432-439.
- Kerckhoffs DA, Brouns F, Hornstra G, Mensink RP. Effects on the human serum lipoprotein profile of beta-glucan, soy protein and isoflavones, plant sterols and stanols, garlic and tocotrienols. J Nutr. 2002 Sep;132(9):2494-2505.
- USDA 2023: United States Department of Agriculture Agricultural Research Service (USDA ARS), Germplasm Resources Information Network (GRIN) – Global. U.S. National Plant Germplasm System. [Accessed 2024 February 1]. Available at: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysearch
References Reviewed
- Clifton PM, Noakes M, Sullivan D, Erichsen N, Ross D, Annison G, Fassoulakis A, Cehun M, Nestel P. 2004. Cholesterol-lowering effects of plant sterol esters differ in milk, yoghurt, bread and cereal. European journal of clinical nutrition. 58: 503-509.
- Davidson, MH, Maki KC, Umporowicz DM, Ingram KA, Dicklin MR, Schaefer E, Lane RW, McNamara JR, Ribaya-Mercado JD, Perrone G, Robins SJ, Franke WC. Safety and Tolerability of Esterified Phytosterols Administered in Reduced-Fat Spread and Salad Dressing to Healthy Adult Men and Women. 2001 Journal of the American College of Nutrition. 20: 307-319.
- Hendriks HF, Brink EJ, Meijer GW, Princen HM, Ntanios FY. 2003. Safety of long-term consumption of plant sterol esters-enriched spread. European journal of clinical nutrition. 57: 681-692.
- Hendriks HFJ, Weststrate JA, Vliet T, Meijer GW. 1999. Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolaemic and mildly hypercholesterolaemic subjects. European journal of clinical nutrition. 53:319-327.
- Lau VW, Journoud M, Jones PJ. Plant sterols are efficacious in lowering plasma LDL and non-HDL cholesterol in hypercholesterolemic type 2 diabetic and nondiabetic persons. American Journal of Clinical Nutrition. 2005:81:1351-1358.
- Thomsen AB, Hansen HB, Christiansen C, Green H, Berger A. Effect of free plant sterols in low-fat milk on serum lipid profile in hypercholesterolemic subjects. Eur J Clin Nutr. 2004 Jun;58(6):860-870.
- Westrate JA, Meijer GW. 1998. Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. European journal of clinical nutrition. 52:334-343.