WILLOW BARK
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization. It is not intended to be a comprehensive review of the medicinal ingredient.
Notes
- Text in parentheses is additional optional information which can be included on the PLA and product label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or statements are synonymous. Either term or statement may be selected by the applicant.
Date
March 28, 2024
Proper name(s), common name(s), Source information
| Proper name(s) | Common name(s) | Source information | ||
|---|---|---|---|---|
| Source material(s) | Part(s) | Preparation(s) | ||
| Willow Bark | Willow Bark |
|
|
Dry |
References: Proper name: Blumenthal et al. 2000, Bradley 1992; Common name: Blumenthal et al. 2000, Bradley 1992; Source information: USDA 2023, EMA 2017, Wichtl 2004, ESCOP 2003, Blumenthal et al. 2000.
Route of administration
Oral
Dosage form(s)
- This monograph excludes foods or food-like dosage forms as indicated in the Compendium of Monographs Guidance Document.
- Acceptable dosage forms for oral use are indicated in the dosage form drop-down list of the web-based Product Licence Application form for Compendial applications.
Use(s) or Purpose(s)
- Used in Herbal Medicine for short-term relief of lower back pain (EMA 2017).
- Used in Herbal Medicine to relieve minor joint pain (due to osteoarthritis) (EMA 2017, ESCOP 2003).
- Used in Herbal Medicine to relieve fever associated with the common cold (EMA 2017).
- Used in Herbal Medicine to relieve headache (EMA 2017).
Notes: The above uses can be combined on the product label (e.g., Used in Herbal Medicine to help relieve fever associated with the common cold and headache).
Dose(s)
Subpopulation(s)
Adults 18 years and older
Quantity(ies)
Note: When 'decoction' or 'infusion' is listed as an acceptable method of preparation, 'decoction concentrate' or 'infusion concentrate' is also allowed. It also applies to standardized methods of preparation.
Methods of preparation: Dry, Powdered, Non-Standardized Extracts (Dry extract, Tincture, Fluid extract, Decoction, Infusion)
3- 9 grams of dried (young branch) bark per day in divided doses: Not to exceed 3 grams per single dose (EMA 2017; ESCOP 2003; Barnes et al. 2002; Blumenthal et al. 2000)
Methods of preparation: Standardized extracts (Dry extract, Tincture, Fluid extract, Decoction, Infusion)
Extract providing up to 15% total salicin equivalent to 45 - 240 milligrams of total salicin per day in divided doses; Not to exceed 120 milligrams of salicin per single dose (EMA 2017; WHO 2009; Wichtl 2004; ESCOP 2003; Barnes et al. 2002; Blumenthal et al. 2000)
Direction(s) for use
No statement required
Duration(s) of use
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 6 weeks (Beer and Wegener 2008; Biegert et al. 2004).
Risk information
Caution(s) and warning(s)
- Ask a health care practitioner/health care provider/health care professional/doctor/physician if symptoms persist or worsen.
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have asthma or peptic ulcer disease (EMA 2017).
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are taking blood thinners or products containing salicylates (e.g., acetylsalicylic acid or non-steroidal anti-inflammatory drugs) (EMA 2017).
Contraindication(s)
- Do not use if you are pregnant or breastfeeding (EMA 2017; Brinker 2010; Wichtl 2004; ESCOP 2003; Barnes et al. 2002; Blumenthal et al. 2000).
- Do not use if you are allergic to salicylates (EMA 2017; Brinker 2010; Wichtl 2004, ESCOP 2003; Barnes et al. 2002; Blumenthal et al. 2000).
Known adverse reaction(s)
When using this product you may experience gastrointestinal discomfort/disturbances (EMA 2017; Brinker 2010; Wichtl 2004; ESCOP 2003; Barnes et al. 2002; Blumenthal et al. 2000)
Non-medicinal ingredients
Must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in the database.Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations.
Specifications
- The finished product specifications must be established in accordance with the requirements described in the Natural and Non-prescription Health Products Directorate (NNHPD) Quality of Natural Health Products Guide.
- The medicinal ingredient must comply with the requirements outlined in the NHPID.
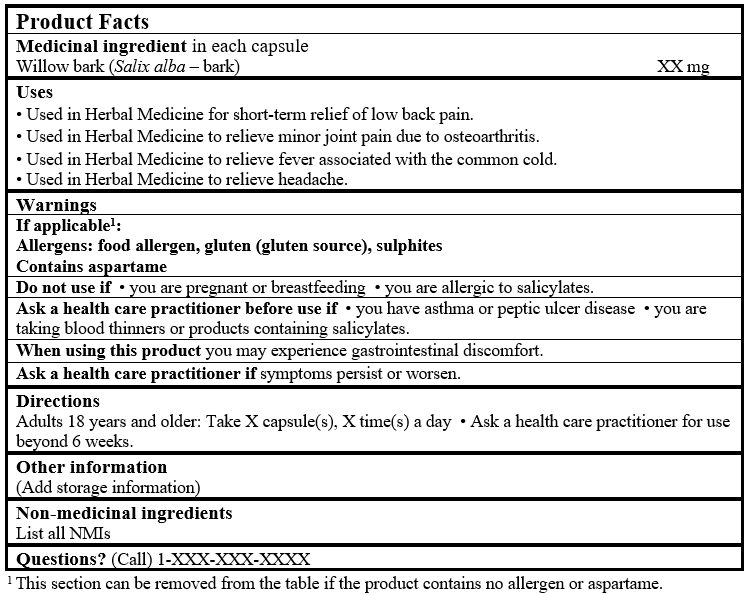
EXAMPLE OF PRODUCT FACTS:
Consult the Guidance Document, Labelling of Natural Health Products for more details.
References cited
- Barnes J, Anderson LA, Philipson JD. Herbal Medicines: A Guide for Healthcare Professionals. 2nd edition. London (GB): The Pharmaceutical Press; 2002.
- Beer A.-M, Wegener T. Willow bark extract (Salicis cortex) for gonarthrosis and coxarthrosis- Results of a cohort study with a control group. Journal of Phytomedicine 2008; 15: 907-913.
- Biegert C, Wagner I, Ludtke R, Kotter I, Lohmuller C, Gunaydin I, Taxis K, Heide L. Efficacy and safety of willow bark extract in the treatment of osteoarthritis and rheumatoid arthritis: results of 2 randomized double-blind controlled trials. Journal of Rheumatology 2004; 31 (11): 2121-2130.
- Blumenthal M, Goldberg A, Brinckmann J, editors. Herbal Medicine: Expanded Commission E Monographs. Newton (MA): Integrative Medicine Communications; 2000.
- Bradley PR, editor. British Herbal Compendium: A Handbook of Scientific Information on Widely Used Plant Drugs, Volume 1. Bournemouth (GB): British Herbal Medicine Association; 1992.
- Barnes J, Anderson LA, Philipson JD. Herbal Medicines: A Guide for Healthcare Professionals. 2nd edition. London (GB): The Pharmaceutical Press; 2002.
- Brinker F. Herb Contraindications and Drug Interactions, 3rd edition. Sandy (OR): Eclectic Medical Publications; 2010.
- Chrubasik S, Eisenberg E, Balan E, Weinberger T, Luzzati R, Conradt C. Treatment of low back pain exacerbations with willow bark extract: a randomised double-blind study. The American Journal of Medicine. 2000; 109: 9-14.
- EMA 2017
 European Medicines Agency.
European Union herbal monograph on Salix [various species including S. purpurea L., S.
daphnoides
Vill., S. fragilis L.], cortex. London (GB): EMA Committee on Herbal Medicinal Products
(HMPC),
31 January 2017. [Accessed 2023 October 14].Available at:
https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-salix-various-species-including-s-purpurea-l-s-daphnoides-vill_en.pdf
European Medicines Agency.
European Union herbal monograph on Salix [various species including S. purpurea L., S.
daphnoides
Vill., S. fragilis L.], cortex. London (GB): EMA Committee on Herbal Medicinal Products
(HMPC),
31 January 2017. [Accessed 2023 October 14].Available at:
https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-salix-various-species-including-s-purpurea-l-s-daphnoides-vill_en.pdf
- ESCOP 2003: E/S/C/O/P Monographs: The Scientific Foundation for Herbal Medicinal Products. 2nd edition. Exeter (GB): ESCOP, the European Scientific Cooperative on Phytotherapy in collaboration with Georg Thieme Verlag and Thieme; 2003.
- USDA 2023:
 United
States Department of Agriculture, Agricultural Research Service, National Genetic Resources
Program.
Germplasm Resources Information Network (GRIN). National Germplasm Resources Laboratory,
Beltsville (MD). [Accessed 2023 October 14]. Available from:
https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysearch
United
States Department of Agriculture, Agricultural Research Service, National Genetic Resources
Program.
Germplasm Resources Information Network (GRIN). National Germplasm Resources Laboratory,
Beltsville (MD). [Accessed 2023 October 14]. Available from:
https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysearch - Wichtl M, editor. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis. 3rd edition. Stuttgart (DE): Medpharm Scientific Publishers; 2004.
- WHO 2009: World Health Organization. WHO Monographs on Selected Medicinal Plants, Volume 4. Geneva (CH): World Health Organization; 2009.