Oral Health Products
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
Date
February 23, 2024
Foreword
This monograph is intended to replace the existing Oral Health Products Monograph of August 26, 2022. This monograph describes the requirements necessary to receive market authorization [i.e. a Drug Identification Number (DIN) or a Natural Product Number (NPN)], for oral health products. The monograph identifies the permitted medicinal and non-medicinal ingredients, concentrations, indications, directions and conditions of use for these products to be market authorized without the submission to Health Canada of additional evidence. It may also contain the test methods recommended to be used to comply with the requirements of this monograph. Products which do not meet the criteria outlined in this document can apply for market authorization outside of the monograph stream.
Applicants are reminded that oral health products, like other non-prescription drugs or natural health products, are subject to the Food and Drug Regulations or the Natural Health Products Regulations administered by the Natural and Non-prescription Health Products Directorate (NNHPD). This includes requirements related to labelling, manufacturing and product specifications. Additional information on labels, outside of those specified in the monograph, such as additional directions for use and/or non-therapeutic claims are acceptable as long as they meet the Guidelines for the Nonprescription and Cosmetic Industry Regarding Non-therapeutic Advertising and Labelling Claims, the Guidelines for Consumer Advertising of Health products for Nonprescription drugs, Natural Health Products, Vaccines and Medical Devices, and are not false, misleading or counterintuitive to the use of the product.
Notes:
- Text in parentheses is additional optional information which can be included on the Product Licence Application form and label at the applicant’s discretion.
- The solidus (/) indicates that the terms and/or the statements are synonymous. Either term or statement may be selected by the applicant.
Medicinal ingredient(s)
Oral health products are classified as natural health products (NHP) if they contain an ingredient listed in Table 1. Applicants seeking to obtain a NPN can access the appropriate forms and guidance at https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription.html.
Oral health products are classified as non-prescription drugs (NPD) if they contain an ingredient from Table 2 at a quantity listed. Applicants applying for a DIN can access the appropriate forms and templates at: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions.html.
| Proper Name1 | Common Name1 | Source information1,2 |
|---|---|---|
| Source ingredient(s) | ||
| Acidulated phosphate fluoride3 | Acidulated phosphate fluoride | Acidulated phosphate fluoride |
|
Sodium monofluorophosphate | Sodium monofluorophosphate |
| Nitric acid potassium salt (1:1) | Potassium nitrate | Potassium nitrate |
| Sodium fluoride | Sodium fluoride | Sodium fluoride |
|
|
Stannous fluoride |
1At least one of the following references was consulted per proper name, common name, and source information: RSC 2024; USP-NF 2023; Nikitakis and Lange 2016.
2Ingredient must be pharmacopoeial grade (for a list of acceptable pharmacopoeial grades, see the Quality of Natural Health Products Guide).
3Acidulated phosphate fluoride is derived from sodium fluoride acidulated with a mixture of sodium phosphate dibasic or monobasic and phosphoric acid to a level of 0.1 molar phosphate to yield a pH of 3.0 to 4.5 (US FDA 2023).
| Proper Name | Common Name | Quantity |
|---|---|---|
| 1-Hexadecylpyridinium chloride | Cetylpyridinium Chloride | 0.05 - 0.075% |
Route(s) of administration
- Dental
- Gingival
- Periodontal
Dosage form(s)
For Natural Health Products
Acceptable dosage forms are as follows:
Dental:
Concentrate; Dental liquid, suspension; Dentifrice, gel; Dentifrice, paste; Gel; Mouthwash; Powder for solution;
Solution; Tablet, effervescent.
Gingival:
Concentrate; Dentifrice, gel; Dentifrice, paste; Gel; Gingival gel; Gingival solution; Mouthwash; Powder for
solution; Solution; Tablet, effervescent.
Periodontal:
Concentrate; Dental liquid, suspension; Dentifrice, gel; Dentifrice, paste; Gel; Gingival gel; Gingival
solution;
Mouthwash; Powder for solution; Solution; Tablet, effervescent.
For Non-prescription drugs
The only acceptable dosage forms for products containing Cetylpyridinium chloride are as follows: Mouthwash; Solution.
Notes:
The above dosage forms correspond to the following product categories:
- Dentifrices: dentifrice, gel; dentifrice, paste
- Preventive treatment gel: gel; gingival gel
- Treatment rinse: dental liquid, suspension; gingival solution; solution
- Concentrated treatment rinse: concentrate; gingival solution; solution; powder for solution; tablet, effervescent
- Mouthwash
Use(s) or Purpose(s)
Self-Care Framework Category I Uses or Purposes:
Products listed in Table 1, excluding Potassium nitrate
- Anti-cavity/Anti-caries (IOM 1997; Zimmerman 1992)
- Prevents, fights and/or protects against cavities/caries (US FDA 2023; Sweetman 2017; Zimmerman 1992).
- Reduces the incidence of cavities/caries (US FDA 2023; Sweetman 2017; Zimmerman 1992)
- Helps prevent tooth decay (US FDA 2023; Sweetman 2017; Zimmerman 1992).
- Effective fluoride protection (US FDA 2023).
- Effective decay preventive fluoride (US FDA 2023).
- Protects teeth from acid wear/erosion (Sweetman 2017; IOM 1997; Zimmerman 1992).
- Helps delay/slow the tooth decay process at the earliest stage before it can become a cavity (IOM 1997).
- Penetrates tooth enamel to help rebuild weak spots (Sweetman 2017; Zimmerman 1992).
- Helps remineralize tooth enamel (Sweetman 2017; Zimmerman 1992).
Products containing Potassium nitrate
- Helps reduce (painful) sensitivity of the teeth (due to cold/heat/acids/sweets/contact) (US FDA 2022; Silverman et al. 1996; Nagata et al. 1994).
- Builds protection/Protects from (painful) sensitivity of the teeth (due to cold/heat/acids/sweets/contact) (US FDA 2022; Palé et al. 2013).
- (Builds)(Effective) Protection against (painful) sensitivity of the teeth (due to cold/heat/acids/sweets/contact) (US FDA 2022; Palé et al. 2013).
- Shields/Soothes dental nerves for lasting sensitivity relief when used regularly (US FDA 2022; Silverman et al. 1996; Nagata et al. 1994;).
Products containing cetylpyridinium chloride
- Kills germs that cause bad breath.
- Helps prevent/reduce plaque and gingivitis.
Dose(s)
Subpopulation(s)
For dentifrice products that do not contain Potassium nitrate
Children 2 to 11 years, Adolescents 12 to 17 years, Adults 18 years and older.
For products that do not contain potassium nitrate in the following dosage forms: Preventive treatment gel; treatment rinse; concentrated treatment rinse, mouthwash (rinse)
Children 6 to 11 years, Adolescents 12 to 17 years, Adults 18 years and older.
For products containing Potassium nitrate
Adolescents 12 to 17 years, Adults 18 years and older.
Quantity(ies)
| Medicinal ingredient | Quantity (% w/w) |
Theoretical Total Fluoride (mg/kg) |
Available Fluoride Ion (mg/kg) |
Frequency |
|---|---|---|---|---|
| Potassium nitrate | 5 | N/A | N/A | minimum 2 times a day |
| Sodium fluoride | 0.188 - 0.254 | 850 - 1150 | ≥ 650 | minimum 2 times a day |
| Sodium monofluorophosphate | 0.654 - 0.884 | 850 - 1150 | ≥ 800 PO3F2- and F- combined | minimum 2 times a day |
| Stannous fluoride | 0.351 - 0.474 | 850 - 1150 | ≥ 700 (for products containing abrasives other than calcium pyrophosphate) ≥ 600 (for products containing the abrasive silica) ≥ 290 (for products containing the abrasive calcium pyrophosphate) |
minimum 2 times a day |
| Medicinal ingredient | Quantity(% w/w) | Frequency |
|---|---|---|
| Acidulated phosphate fluoride | 0.01 | 2 times a day |
| 0.02 | 1 time a day | |
| Potassium nitrate | 3 | 2 times a day |
| Sodium Fluoride | 0.02 | 2 times a day |
| 0.05 | 1 time a day | |
| 0.21 | 1 time a week | |
| Sodium fluoride (as concentrated treatment rinse)2 | Diluted in water to 0.02 | 2 times a day |
| Diluted in water to 0.05 | 1 time a day | |
| Diluted in water to 0.2 | 1 time a week | |
| Stannous fluoride (in preventive treatment gel only) | 0.4 | 1 time a day |
| Stannous fluoride (as concentrated treatment rinse)2 | diluted in water to 0.1 | 1 time a day |
1US FDA 2023, Sharma et al. 2012, Sweetman 2017, Gillam 1996.
2For concentrated treatment rinse products requiring dilution: The concentration of the product before dilution, the final concentration of the rinse once diluted and appropriate directions for use for each dilution explaining how to dilute the product must be included on the Product Licence Application form and label. The final concentration of the rinse and the associated frequency of use must meet the information listed in Table 4.
End-of shelf life minimal Fluoride Ion (mg/kg= ppm)
- Sodium fluoride: ≥ 403 mg/kg
- Sodium monofluorophosphate : ≥ 600 mg/kg
- Stannous fluoride: ≥ 650 mg/kg for products containing abrasives other than calcium pyrophosphate; ≥ 108 mg/kg for products containing the abrasive calcium pyrophosphate
Note: End of Shelf life minimal fluoride Ion values in mg/kg can be included as additional information on the label but are not required on the PLA. These values are intended for determining expiration dating and are not to be used for determining safety and efficacy
Permitted combinations
Potassium nitrate can be combined with any one of the ingredient listed in Table 1. Any other combination of medicinal ingredients should be assessed outside of the compendial stream.
Directions for use
For all products, the following statement must be made
- Do not swallow.
For all products, the following statement may be made
- Should be used as/Use as part of an oral health program that includes regular flossing and dental check-ups (CDA 2018).
For dentifrice products, the following statement must be made
- Brush teeth thoroughly (for at least 1 minute), preferably after each meal, or as directed by a dentist/health care practitioner/health care provider/health care professional/doctor/physician (US FDA 2023).
For dentifrice products that do not contain Potassium nitrate, the following statement must be made when the subpopulation includes children under 6 years
- Children under 6 years of age should use only a pea-sized amount and be supervised (to brush properly and to not swallow) (CDA 2018).
For dentifrice products that do not contain Potassium nitrate, the following statement may be made when the subpopulation includes children
- Ask a dentist/health care practitioner/health care provider/health care professional/doctor/physician before using in children under 2 years of age.
For preventive treatment gels, treatment rinses, concentrated treatment rinses, and mouthwash (rinse) products, the following statements must be made
- Use after brushing (teeth) with toothpaste (US FDA 2023; Krinsky 2017).
- Do not eat, drink or rinse with water for 30 minutes after use (US FDA 2023; Krinsky 2017).
For preventive treatment gels, treatment rinses, concentrated treatment rinses, and mouthwash (rinse) products, the following statement must be made when the subpopulation includes children under 12 years
- Instruct children under 12 years of age (in the proper use of the product and) in good brushing and rinsing habits to minimize swallowing (US FDA 2023; Krinsky 2017).
For daily treatment rinses, concentrated treatment rinses, and mouthwash (rinse) products containing 0.02% and 0.05% sodium fluoride, the following statement may be made when the subpopulation includes children
- Ask a dentist/health care practitioner/health care provider/health care professional/doctor/physician before using in children under 6 years of age (US FDA 2023; Krinsky 2017).
For weekly rinses with 0.2% sodium fluoride, the following statements must be made when the subpopulation includes children
- Not recommended for use in children under 6 years of age (Sweetman 2017).
For preventive treatment gel products, the following statement must be made
- Apply the gel to teeth and brush thoroughly. Allow the gel to remain on the teeth for 1 minute and then spit out (US FDA 2023; Krinsky 2017).
For treatment rinses, concentrated treatment rinses (after proper dilution), and mouthwash (rinse) products excluding Cetylpyridinium chloride, the following statement must be made
- Swish approximately 10 mL of rinse vigorously around and between the teeth for 1 minute and then spit out (US FDA 2023; Krinsky 2017).
For concentrated treatment rinse products in a solution, effervescent tablet, or powder dosage form, the following statements must be made
Sodium fluoride
Do not use before diluting with water and until the tablet/powder has dissolved completely/the solution has been fully mixed. Dilute the product to obtain a final concentration of 0.02% according to the following instructions and rinse mouth twice a day. [Insert instructions]. Use immediately after preparing the rinse (US FDA 2023).
And/or
Do not use before diluting with water and until tablet/powder has dissolved completely/the solution has been fully mixed. Dilute the product to obtain a final concentration of 0.05% according to the following instructions and rinse mouth once a day. [Insert instructions]. Use immediately after preparing the rinse (US FDA 2023).
And/or
Do not use before diluting with water and until tablet/powder has dissolved completely/the solution has been fully mixed. Dilute the product to obtain a final concentration of 0.2% according to the following instructions and rinse mouth once a week. [Insert instructions]. Use immediately after preparing the rinse (US FDA 2023).
Stannous fluoride
Do not use before diluting with water and until tablet/powder has dissolved completely/the solution has been fully mixed. Dilute the product to obtain a final concentration of 0.1% according to the following instructions and rinse mouth once a day. [Insert instructions]. Use immediately after preparing the rinse (US FDA 2023).
Note:
Instructions: Additional directions for use explaining appropriate dilution of the product must be included to the Directions for use.
For products containing potassium nitrate, the following statement must be made when the subpopulation includes adolescents
- Not recommended for children under 12 years of age (US FDA 2023).
For products containing Cetylpyridinium chloride, the following statements must be made
- Use after (your normal) brushing and flossing (routine). Rinse toothpaste from mouth prior to use.
- Rinse/Swish/Gargle around the mouth twice a day for 30 seconds with 20 mL (4 teaspoonfuls) and spit out.
- Do not eat or drink anything for 30 minutes after use.
For products containing Cetylpyridinium chloride, the following statements may be made:
- (This product is not intended to replace brushing or flossing).
Duration(s) of use
No statement is required.
Risk information
Caution(s) and warning(s)
All products
- Keep out of reach of children. If swallowed, call a poison control centre or get medical help right away.
Preventative treatment gels products containing stannous fluoride
- When using this product surface staining of the teeth may occur. Adequate brushing may prevent these stains which are harmless and temporary and may be removed by a dentist/dental care practitioner (US FDA 2023).
Products containing potassium nitrate
- Stop use and ask a dentist/dental care practitioner/health care practitioner/health care provider/health care professional/doctor/physician if symptoms persist or worsen (US FDA 2023)
Products containing cetylpyridinium chloride
- When using this product surface staining of the teeth may occur. Adequate brushing may prevent these stains which are harmless and temporary and may be removed by a dentist/dental care practitioner.
Products containing more than the equivalent of 120 mg of fluoride ion, except those in toothpaste form (includes dentifrice paste and dentifrice gel)
- Keep out of reach of children. This product contains enough fluoride to seriously harm a child. (Note: These cautionary statements shall be preceded by a prominently displayed symbol that is octagonal in shape, conspicuous in colour and on a background of a contrasting colour. If the product is recommended solely for children, all package sizes must be packaged in a child-resistant package (CRP). If the product is not recommended solely for children, at least one of the sizes of packages available for sale must be packaged in CRP and all other package sizes must carry a statement that the NHP is available in a CRP, as per Section 97 of the Natural Health Product Regulations, citing Sections C.01.029, C.01.031 and C.01.031.2 (1) of the Food and Drug Regulations (JC 2023)).
Contraindication(s)
Products containing cetylpyridinium chloride
- Do not use if you have painful or swollen gums, pus from the gum line, loose teeth or increased spacing between the teeth. See your dentist immediately, as these may be signs of periodontitis, a serious gum disease.
Non-medicinal ingredients
Ingredients must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in that database, the Food and Drug Regulations (FDR), and the current Cosmetic Ingredient Hotlist, when relevant.
Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations or the Food and Drug Regulations.
Specifications
This monograph describes those requirements that are specific to this class of non-prescription drugs and to NHPs. Any change to the manufacturing process that impacts the safety and efficacy of the ingredients, such as the use of novel technology (e.g. nanotechnology), requires supporting data and will be reviewed outside the monograph.
For products containing Table 1 NHP medicinal ingredients only:
The finished product specifications must be established in accordance with the requirements described in the NNHPD Quality of Natural Health Products Guide. The medicinal ingredient must comply with the requirements outlined in the NHPID.
The medicinal ingredient(s) must either comply with the specifications outlined in the monographs published in the British(BP), European (Ph. Eur.) or United States(USP) pharmacopoeias; or, be cited in an approved NHP Master File, authorized by a letter of access issued to the applicant by the NHP Master File's registered owner.
Diethylene glycol (DEG) is not acceptable as a non-medicinal ingredient. Product licence applicants must have a copy of a Certificate of Analysis or any other equivalent document confirming the absence of DEG in the finished product on file. This information is not to be submitted with the compendial Product Licence Application, although it may be requested at the NNHPD's discretion.
Products containing glycerin as a non-medicinal ingredient must meet the specifications as outlined in the United States Pharmacopoeia.
For products containing Table 2 NPD medicinal ingredient:
Requirements described in the Regulations to the Food and Drugs Act must be met.
For products containing Table 1 NHP medicinal ingredients only:
EXAMPLE OF PRODUCT FACTS:
Consult the Guidance Document, Labelling of Natural Health Products for more details.
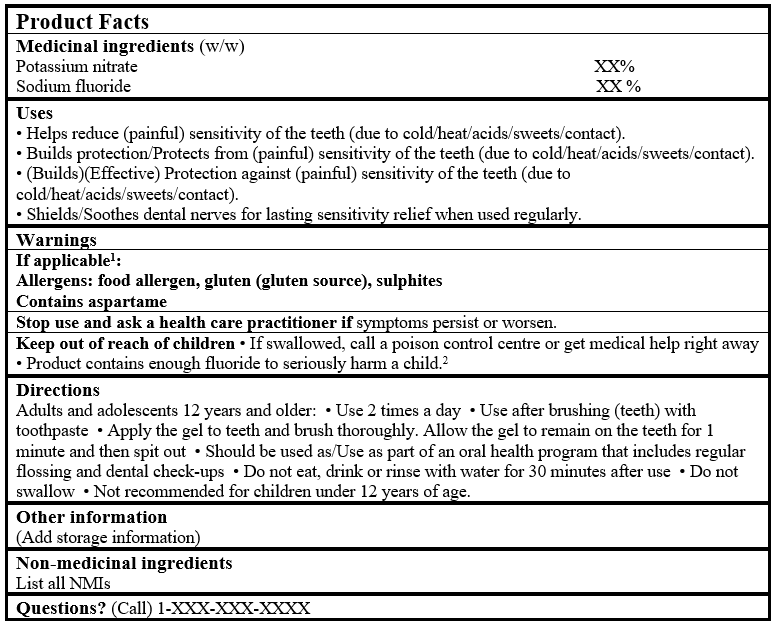
1This section can be removed from the table if the product contains no allergen or aspartame.
2For products containing more than the equivalent of 120 mg of fluoride ion, except those in toothpaste form (includes dentifrice paste and dentifrice gel).
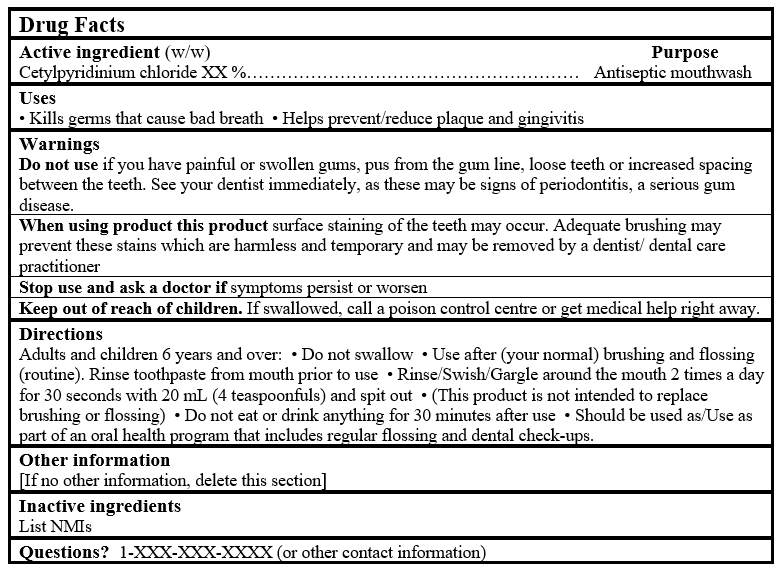
For products containing Table 2 NPD medicinal ingredient:
DRUG FACTS TABLE (Format Optional for Self-Care Category I)
References
- CDA 2018: Canadian Dental Association. 5 Steps to Good Oral Health. Ottawa (ON): Canadian Dental Association. https://www.cda-adc.ca/en/oral_health/cfyt/good_for_life/
- Gillam DG, Bulman JS, Jackson RJ, Newman HN. Efficacy of a potassium nitrate mouthwash in alleviating cervical dentine sensitivity. Journal of Clinical Periodontology 1996; 23:993-997.
- Nikitakis J, Lange B, editors. International Cosmetic Ingredient Dictionary and Handbook. 16th edition. Washington (DC): Cosmetic, Toiletry and Fragrance Association; 2016
- IOM 1997: Institute of Medicine. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): National Academy Press;1997.
- JC 2023: Department of Justice Canada. Food and Drug Regulations. Sections C.01.029, C.01.031 and C.01.031.2 (1). Cautionary Statements and Child Resistant Packages. Ottawa (ON): Department of Justice Canada. [Regulations are current to 2023-11-27; Last amended on 2023-11-24]. [Accessed 2024 January 16]. Available from: http://laws-lois.justice.gc.ca/eng/regulations/SOR-2003-196/
- Nagata T, Ishida H, Shinohara H, Nishikawa S, Kasahara S, Wakano Y, Daigen S, Troullos ES. Clinical evaluation of potassium nitrate dentifrice for the treatment of dentinal hypersensitivity. Journal of Clinical Periodontology 1994;21(3):217-221.
- Palé M, Mayoral JR, Llopis J, Vallès M, Baislio J, Roig M. Evaluation of the effectiveness of an in-office bleaching system and the effect of potassium nitrate as a desensitizing agent. Odontology 2013;102:203-210.
- Sharma S, Shetty NJ, Uppoor A. Evaluation of the clinical efficacy of potassium nitrate desensitizing mouthwash and a toothpaste in the treatment of dentinal hypersensitivity. Journal of Clinical and Experimental Dentistry 2012;4(1):e28-e33.
- Silverman G, Berman E, Hanna CB, Salvato A, Fratarcangerlo P, Bartizek RD, Bollmer BW, Campbell SL, Lanzalaco AC, Mackay BJ, McClanahan SF, Perlich MA, Shaffer JB. Assessing the efficacy of three dentifrices in the treatment of dentinal hypersensitivity. The Journal of the American Dental Association 1996;127(2):191-201.
- Krinsky DL, Ferreri SP, Hemstreet B, Hume AL, Newton GD, Rollins CJ, Tietze KJ, Handbook of Nonprescription Drugs: An interactive approach for Self-Care, 19th edition. Washington (DC): American Pharmaceutical Association; 2017 Prevention of hygiene-related oral disorders, Melody K – Chapter 31.
- RSC 2024: Royal Society of Chemistry: The Merck Index Online [Accessed 2024 January 16]. Available from: https://merckindex.rsc.org/
- Sweetman SC, editor. Martindale: The Complete Drug Reference, 39th edition. Grayslake (IL): Pharmaceutical Press; 2017.
- US FDA 2022: United States Food and Drug Administration. Oral Health Care Drug Products for Over-the-Counter Human Use; United States Department of Health and Human Services, U.S. food and Drug Administration. [Accessed 2024 January 16]. Available from: https://dps-admin.fda.gov/omuf/sites/omuf/files/monograph-documents/2022-11/OTC%20Monograph_M022-Oral%20Healthcare%20Products%20for%20OTC%20human%20Use%2010.14.2022.pdf
- US FDA 2023: United States Food and Drug Administration. Anticaries Drug Products for Over-the-Counter Human Use; United States Department of Health and Human Services, U.S. Food and Drug Administration. [Accessed 2024 January 16]. Available from: https://dps-admin.fda.gov/omuf/sites/omuf/files/monograph-documents/2023-05/OTC%20Monograph%20M021-Anticaries%20Drug%20Products%20for%20OTC%20Human%20Use%2005.02.2023.pdf
- USP-NF 2023: United States Pharmacopeia and the National Formulary. Rockville (MD): United States Pharmacopeial Convention, Inc.; (2023)
- Zimmerman DR. Zimmerman's Complete Guide to Nonprescription Drugs, 2nd edition. Detroit (MI): Gale Research Inc.; 1992.