CARBON DIOXIDE-RELEASING LAXATIVES
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization. It is not intended to be a comprehensive review of the medicinal ingredients.
Notes
- Text in parentheses is additional optional information which can be included on the PLA and product label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or the statements are synonymous. Either term or statement may be selected by the applicant.
Date
February 23, 2024
Proper name(s), Common name(s), Source information
| Proper Name(s) | Common Name(s) | Source information1 |
|---|---|---|
| Source ingredient(s) | ||
| (2R,3R)-2,3-Dihydroxybutanedioic acid potassium salt (1:1) |
|
Potassium bitartrate |
| Carbonic acid sodium salt (1:1) |
|
Sodium bicarbonate |
| Disodium dihydrogen pyrophosphate |
|
Disodium pyrophosphate |
|
|
|
1The ingredients must be pharmacopoeial grade.
References: Proper names: RSC 2024; USP-NF 2023; Gottschalck and McEwen 2006; Sweetman 2002; Common
names: RSC 2024; USP-NF 2023; Gottschalck and McEwen 2006; Sweetman 2002; Source information: RSC 2024;
USP-NF 2023; Gottschalck and McEwen 2006; Sweetman 2002.
Route of administration
Rectal (US FDA 2023).
Dosage form(s)
Suppository (US FDA 2023).
Use(s) or Purpose(s)
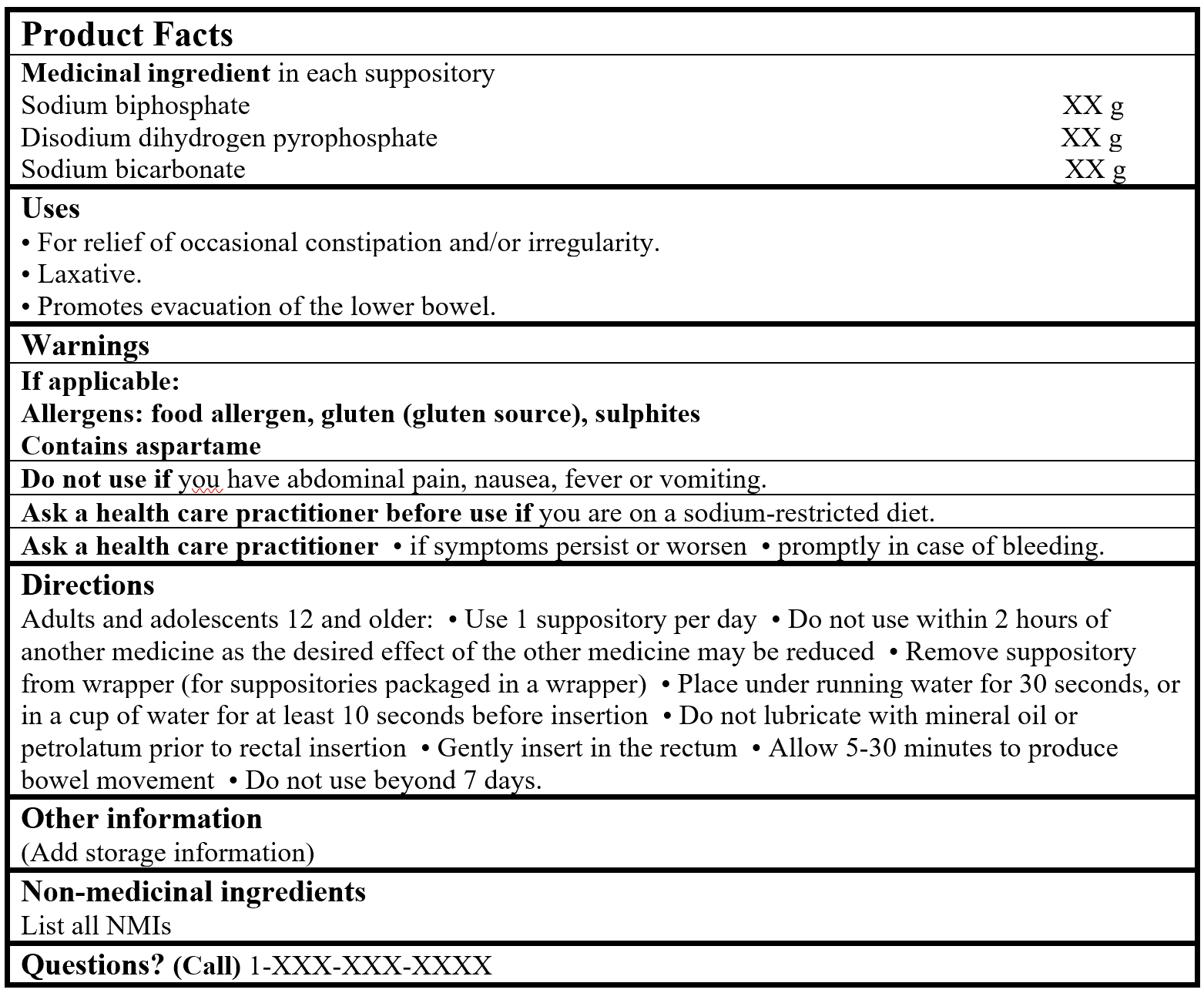
- For relief of occasional constipation and/or irregularity (US FDA 2023).
- Laxative (US FDA 2023).
- Promotes evacuation of the lower bowel (Sweetman 2002).
Note: The above uses can be combined on the product label (e.g. Laxative for the relief of occasional constipation and/or irregularity and promotes evacuation of the lower bowel).
Dose(s)
Subpopulation(s)
Adolescents 12-17 years and Adults 18 years and older (US FDA 2023).
Quantity(ies)
- 1 suppository per day, containing 1.2 - 1.5 grams of Monosodium phosphate + 0.04 -0.05 grams of Disodium dihydrogen pyrophosphate + 1 - 1.5 grams of Carbonic acid sodium salt (1:1) (Sodium bicarbonate) (US FDA 2023).
- 1 suppository per day, containing 0.6 grams of Carbonic acid sodium salt (1:1) (Sodium bicarbonate) + 0.9 grams of (2R,3R)-2,3-Dihydroxybutanedioic acid potassium salt (1:1) (Potassium bitartrate) (US FDA 2023).
Permitted combinations
Products with only one medicinal ingredient are not allowed; medicinal ingredients are permitted only in the combinations indicated in the Quantities section (US FDA 2023).
Direction(s) for use
- Do not use within 2 hours of another medicine as the desired effect of the other medicine may be reduced (Berardi et al. 2002).
- Remove suppository from wrapper (for suppositories packaged in a wrapper) (US FDA 2021).
- Place under running water for 30 seconds, or in a cup of water for at least 10 seconds before insertion (US FDA 2023).
- Do not lubricate with mineral oil or petrolatum prior to rectal insertion (US FDA 2023).
- Gently insert in the rectum.
- Allow 5-30 minutes to produce bowel movement (US FDA 2023).
Duration(s) of use
Do not use beyond 7 days (US FDA 2023).
Risk information
Caution(s) and warning(s)
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are on a sodium-restricted diet (US FDA 2024).
- Ask a health care practitioner/health care provider/health care professional/doctor/physician if symptoms persist or worsen (US FDA 2021).
- Ask a health care practitioner/health care provider/health care professional/doctor/physician promptly in case of bleeding (US FDA 2021).
Contraindication(s)
Do not use if you have abdominal pain, nausea, fever or vomiting (US FDA 2023).
Known adverse reaction(s)
No statement required.
Non-medicinal ingredients
Must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in the database.
Storage Conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations.
Specifications
- The finished product specifications must be established in accordance with the requirements described in the Natural and Non-prescription Health Products Directorate (NNHPD) Quality of Natural Health Products Guide.
- The medicinal ingredient must comply with the requirements outlined in the NHPID.
EXAMPLE OF PRODUCT FACTS:
Consult the Guidance Document, Labelling of Natural Health Products for more details.
References cited
- Berardi RR, DeSimone EM, Newton GD, Oszko MA, Popovich NG, Rollins CJ, Shimp LA, Tietze KJ, editors. Handbook of Nonprescription Drugs: An Interactive Approach to Self-care. 13th edition. Washington (DC): American Pharmaceutical Association; 2002.
- Gottschalck TE, McEwen GN, editors. International Cosmetic Ingredient Dictionary and Handbook. 10th edition. Washington (DC): Cosmetic, Toiletry and Fragrance Association; 2006.
- RSC 2024: Royal Society of Chemistry: The Merck Index Online [Accessed 2024 January 12]. Available from: https://merckindex.rsc.org/
- Sweetman SC, editor. Martindale: The Complete Drug Reference. 33rd edition. Grayslake (IL): Pharmaceutical Press; 2002.
- US FDA 2021. USA Department of Health and Human Services: Food and Drug Administration. Anorectal Drug Products for Over-the-Counter Human Use; 2021. [Accessed 2024 January 12]. Available at: https://dps-admin.fda.gov/omuf/sites/omuf/files/monograph-documents/2022-09/OTC%20Monograph_M015-Anorectal%20Drug%20Products%20for%20OTC%20Human%20Use%20Posted%20Corrected%2002_23_22.pdf
- US FDA 2023. USA Department of Health and Human Services: Food and Drug Administration. Laxative Drug Products for Over-the-Counter Human Use; 2023. [Accessed 2024 January 12]. Available at: https://dps-admin.fda.gov/omuf/sites/omuf/files/monograph-documents/2023-05/OTC%20Monograph_M007-Laxative%20Drug%20Products%20for%20OTC%20Human%20Use%2005.02.2023.pdf
- US FDA 2024: USA Department of Health and Human Services: Food and Drug Administration. 21 CFR Part 201. Code of Federal Regulations; Labeling Requirements for Over-the-Counter Drugs; 2024. [Accessed 2024 January 12]. Available at: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-C/part-201/subpart-C
- USP-NF 2023: United States Pharmacopeia and the National Formulary. Rockville (MD): United States Pharmacopeial Convention, Inc.; 2023.