AFRICAN WILD MANGO - IRVINGIA GABONENSIS
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization. It is not intended to be a comprehensive review of the medicinal ingredient.
Notes
- Text in parentheses is additional optional information which can be included on the PLA and product label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or statements are synonymous. Either term or statement may be selected by the applicant.
Date
December 29, 2023
Proper name(s), Common name(s), Source information
| Proper name(s) | Common name(s) | Source information | ||
|---|---|---|---|---|
| Source material(s) | Part(s) | Preparation | ||
| Irvingia gabonensis | African wild mango | Irvingia gabonensis | Seed | Dry |
References: Proper name: USDA 2023; Common name: USDA 2023; Source information: Ross 2011, Ngondi et al. 2009, Oben et al. 2008a, b, Ekpo et al. 2007, Ngondi et al. 2005.
Route of Administration
Oral
Dosage Form(s)
This monograph excludes foods or food-like dosage forms as indicated in the Compendium of Monographs Guidance Document.
Acceptable dosage forms for oral use are indicated in the dosage form drop-down list of the web-based Product Licence Application form for Compendial applications.
Use(s) or Purpose(s)
- Could be a complement to a healthy lifestyle that incorporates a calorie-reduced diet and regular physical activity for individuals involved in a weight management program (Ross 2011; Ngondi et al. 2009, Ngondi et al. 2005).
- Helps support healthy cholesterol levels/cardiovascular health by reducing total and LDL cholesterol (Ross 2011; Ngondi et al. 2009; Ngondi et al. 2005).
- Helps support healthy glucose levels (Ross 2011; Ngondi et al. 2009; Adamson et al. 1986).
- Source of antioxidants/Provides antioxidants (Atawodi 2011; Agbor et al. 2005).
- Source of antioxidants/Provides antioxidants that help fight/protect (cell) against/reduce (the oxidative effect of/the oxidative damage caused by/cell damage caused by) free radicals (Atawodi 2011; Agbor et al. 2005).
Note: The above claims can be combined on the product label (e.g. Helps support healthy cholesterol levels/cardiovascular health by reducing total and LDL cholesterol and healthy glucose levels).
Dose(s)
Subpopulation(s)
Adults 18 years and older
Quantity(ies)
Weight management, cholesterol, glucose
Method of preparation: Standardized Dry Extracts
150 milligrams of dry extract standardized to 7% albumin, two times per day (Ross 2011; Ngondi et al. 2009; Oben et al. 2008a, b).
Antioxidant
Methods of preparation: Dry, Powder, Non-Standardized Extracts (Dry extract, Tincture, Fluid extract, Decoction, Infusion)
Not to exceed 3.15 grams of dried seed, per day (Ekpe et al. 2007; Ekpo et al. 2007; Ngondi et al. 2005).
Direction(s) for use
Weight management, cholesterol, glucose
Take before meals (Ross 2011; Ngondi et al. 2009; Oben et al. 2008a,b; Ngondi et al. 2005; Adamson et al. 1986).
Duration(s) of Use
No statement required.
Risk Information
Caution(s) and warning(s)
All products
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are breastfeeding.
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have diabetes (Ross 2011; Ngondi et al. 2009; Adamson et al. 1986).
Antioxidant, cholesterol, glucose
Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are pregnant.
Contraindication(s)
Weight management
Do not use if you are pregnant.
Known adverse reaction(s)
Stop use if you experience symptoms of hypoglycaemia including feelings of anxiety, dizziness, tremor, sweating, nausea or headache (Adamson et al. 1986; Ngondi et al. 2009; Oben et al. 2008a,b).
Non-medicinal ingredients
Must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in the database.
Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations.
Specifications
- The finished product specifications must be established in accordance with the requirements described in the Natural and Non-prescription Health Products Directorate (NNHPD) Quality of Natural Health Products Guide.
- The medicinal ingredient must comply with the requirements outlined in the NHPID.
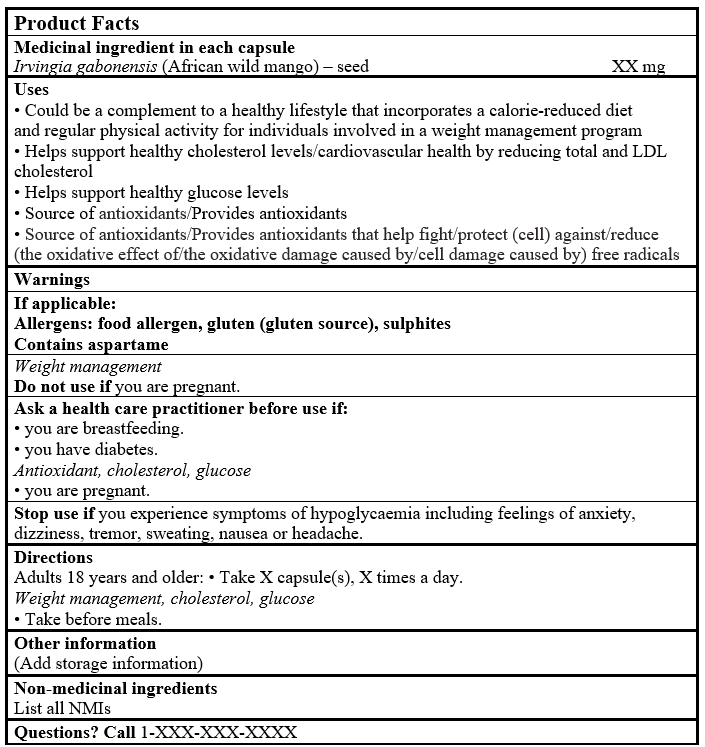
EXAMPLE OF PRODUCT FACTS:
Consult the Guidance Document, Labelling of Natural Health Products for more details.
References Cited
- Adamson I, Okafor C, Abu-Bakare A. Erythrocyte membrane ATPases in diabetes: effect of dikanut (Irvingia gabonensis). Enzyme 1986; 36(3): 212-215.
- Agbor, GA, Oben JE, Ngogang JY, Xinxing C, Vinson JA. Antioxidant capacity of some herbs/spices from Cameroon: a comparative study of two methods. Journal of Agricultural and Food Chemistry 2005; 53(17): 6819-6824.
- Atawodi, SE. Polyphenol content and in vitro antioxidant activity of methanol extract of seeds of Irvingia gabonensis Baill. of Nigerian origin. Electronic Journal of Environmental, Agricultural and Food Chemistry 2011; 10(6): 2314-2321.
- Ekpe OO, Umoh IB, Eka OU. Effect of a typical rural processin method on the proximate composition and amino acid profile of bush mango seeds (Irvingia gabonensis). African Journal of Food, Agriculture, Nutrition and Development. 2007; 7(1): 1684-5374.
- Ekpo IW, Amor ID, Morah FNI. Seed oils and nutritive studies on the seeds of Gabonensis and Wombolu varieties of Irvingia gabonensis. The Nigerian Academic Forum 2007; 13(1): 1-137.
- Ngondi JL, Oben JE, Minka SR. The effect of Irvingia gabonensis seeds on body weight and blood lipids of obese subjects in Cameroon. Lipids in Health and Disease 2005; 4:12.
- Ngondi JL, Etoundi BC, Nyangono CB, Mbofung CM, Oben JE. IGOB131, a novel seed extract of the West African plant Irvingia gabonensis, significantly reduces body weight and improves metabolic parameters in overweight humans in a randomized double-blind placebo controlled investigation. Lipids in Health and Disease 2009; 8:7.
- Oben JE, Ngondi JL, Blum K. Inhibition of Irvingina gabonensis seed extract (OB131) on adipogenesis as mediated via down regulation of the PPARgamma and leptin genes and up- regulation of the adiponectin gene. Lipids in Health and Disease 2008a; 7:44.
- Oben JE, Ngondi JL, Momo CL, Agbor GA, Makamto Sobgui CS. The use of a Cissus quadrangularis/Irvingia gabonensis combination in the management of weight loss: a double- blind placebo-controlled study. Lipids in Health and Disease 2008b; 7:12.
- Ross MS. A proprietary seed extract of Irvingia gabonensis is found to be effective in reducing body weight and improving metabolic parameters in overweight humans. Holistic Nursery Practice 2011; 235(4): 215-217.
- USDA 2023: United States Department of Agriculture Agricultural Research Service (USDA ARS), Germplasm Resources Information Network (GRIN) – Global. U.S. National Plant Germplasm System. [Accessed 2023 September 11]. Available from: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysearch
References Reviewed
- Adamson I, Okafor C, Abu-Bakare A. A supplement of Dikanut (Irvingia gabonensis) improves treatment of type II diabetics. West African Journal of Medicine 1990; 9(2): 108-115.
- Kothari SC, Shivarudraiah P, Venkataramaiah SB, Gavara S, Soni MG. Subchronic toxicity and mutagenicity/genotoxicity studies of Irvingia gabonensis extract (IGOB131). Food and Chemical Toxicology 2012; 50: 1468-1479.
- Leung, Woot-tsuen Wu. & Leung, Woot-tsuen Wu. & Food and Agriculture Organization of the United Nations. Food Consumption and Planning Branch. & United States. Nutrition Program. Food composition table for use in Africa; a research project sponsored jointly by U.S. Dept. of Health, Education, and Welfare, Nutrition Program, and Food Consumption and Planning Branch, Food and Agriculture Organization of the United Nations. Bethesda, Md 1968.
- Onakpoya I, Davies L, Posadzki P, Ernst E. The efficacy of Irvingia gabonensis supplementation in the management of overweight and obesity: A systematic review of randomized controlled trials. Journal of Dietary Supplements 2013; 10(1): 29-38.