PANAX GINSENG
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization. It is not intended to be a comprehensive review of the medicinal ingredient.
Notes
- Text in parentheses is additional optional information which can be included on the PLA and product label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or statements are synonymous. Either term or statement may be selected by the applicant.
Date
February 23, 2024
Proper name(s), Common name(s), Source information
| Proper name(s) | Common name(s) | Source information | ||
|---|---|---|---|---|
| Source material(s) | Part(s) | Preparation(s) | ||
| Panax ginseng |
|
Panax ginseng |
|
Dry |
References: Proper name: USDA 2024; Gardner and McGuffin 2013; Common names: USDA 2024; USP-NF 2023; PPRC 2015; Gardner and McGuffin 2013; Vuksan et al. 2008; Reay et al. 2006; Kim et al. 2005; Sünram-Lea et al. 2005; WHO 1999; Source information: USP-NF 2023; PPRC 2015; Ph. Eur 2008; Vuksan et al. 2008; Sievenpiper et al. 2006; ESCOP 2003; WHO 1999; Bradley 1992; BHP 1983; Wren 1907.
Route of Administration
Oral
Dosage Form(s)
This monograph excludes foods or food-like dosage forms listed as indicated in the Compendium of Monographs Guidance Document.
Acceptable dosage forms for oral use are indicated in the dosage form drop-down list of the web-based Product Licence Application form for Compendial applications.
Use(s) or Purpose(s)
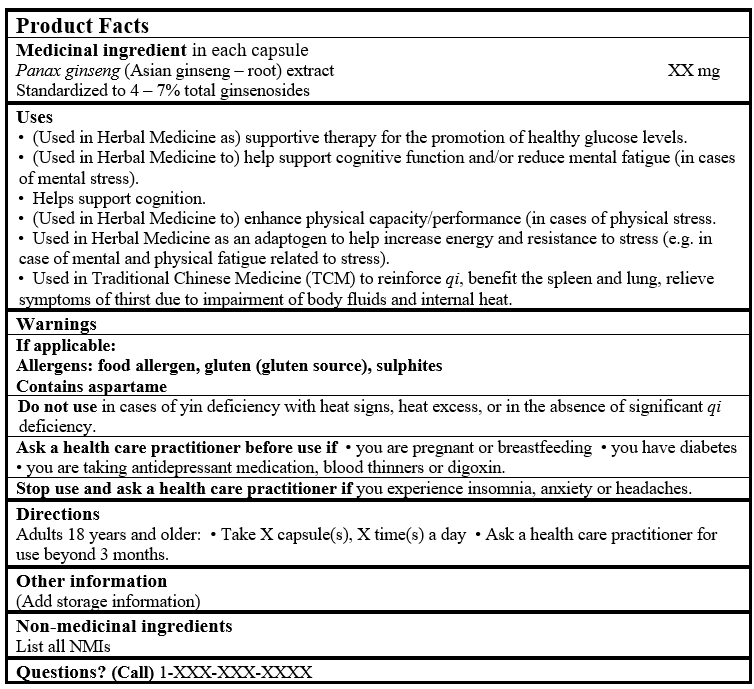
- (Used in Herbal Medicine as) supportive therapy for the promotion of healthy glucose levels (Vuksan et al. 2008; Sievenpiper et al. 2006; Williamson 2003; Tetsutani et al. 2000; WHO 1999; Sotaniemi et al. 1995).
- (Used in Herbal Medicine to) help(s) support cognitive function and/or reduce mental fatigue (in cases of mental stress) (Reay et al. 2006; Reay et al. 2005; Sünram-Lea et al. 2005; Kennedy et al. 2004; ESCOP 2003; Kennedy et al. 2002; Scholey and Kennedy 2002; Kennedy et al. 2001; Sorensen and Sonne 1996; Bradley 1992; D'Angelo et al. 1986; BHP 1983).
- Helps support cognition (Reay et al. 2006; Reay et al. 2005; Sünram-Lea et al. 2005; Kennedy et al. 2004; ESCOP 2003; Kennedy et al. 2002; Scholey and Kennedy 2002; Kennedy et al. 2001; Sorensen and Sonne 1996; Bradley 1992; D'Angelo et al. 1986; BHP 1983).
- (Used in Herbal Medicine to) help(s) enhance physical capacity/performance (in cases of physical stress) (Kim et al. 2005; ESCOP 2003; Gross et al. 2002; WHO 1999; Gross et al. 1995; Sotaniemi et al. 1995; Schepdael 1993).
- Used in Herbal Medicine as an adaptogen to help increase energy and resistance to stress (e.g. in case of mental and physical fatigue related to stress) (Winston and Maimes 2007, Bone 2003, Hoffman 2003, Blumenthal et al. 2000, WHO 1999, Bradley 1992).
- Used in Traditional Chinese Medicine (TCM) to reinforce qi, benefit the spleen and lung, relieve symptoms of thirst due to impairment of body fluids and internal heat (PPRC 2015; Bensky et al. 2004, Chen and Chen 2004).
Notes
- The above uses can be combined on the product label if from the same traditional or non-traditional system of medicine (e.g. Used in Herbal Medicine to help support cognitive function and/or reduce mental fatigue and as an adaptogen to help increase energy and resistance to stress.)
- For multi-ingredient products:
-
- To prevent the product from being represented as a “traditional medicine”, any indicated traditional use claim must refer to the specific medicinal ingredient(s) and recognized system of medicine from which the claim originates when 1) both the traditional and modern claims are present or 2) when claims original from multiple systems of traditional medicine (e.g. Panax Ginseng is used in Traditional Chinese Medicine to reinforce qi, benefit the spleen and lung, relieve symptoms of thirst due to impairment of body fluids and internal heat).
- When ALL of the medicinal ingredients (MIs) in the product are used within the SAME identified system of traditional medicine AND the product makes ONLY traditional claims, listing of MIs in the traditional claim(s) is not required.
Dose(s)
Subpopulation(s)
Adults 18 years and older
Quantity(ies)
Glucose levels; Cognitive function/cognition; Mental stress; Physical stress; AdaptogenMethods of preparation: Standardized extracts (Dry extract)
200 - 600 milligrams of extract, per day; standardized to 4 - 7% total ginsenosides; Not to exceed 9 grams of dried root/rootlet, per day (Vuksan et al. 2008; Reay et al. 2006; Sievenpiper et al. 2006; Reay et al. 2005; Sünram-Lea et al. 2005; Kennedy et al. 2004; ESCOP 2003; Kennedy et al. 2002; Scholey and Kennedy 2002; Engels et al. 2001; Kennedy et al. 2001; Scaglione et al. 2001; Blumenthal et al. 2000; Tetsutani et al. 2000; Engels et al. 1996; Scaglione et al. 1996; Gross et al. 1995; Scaglione et al. 1994; Scaglione et al. 1990; Petkov and Mosharrof 1987; D'Angelo et al. 1986; Soldati and Sticher 1980).
Methods of preparation: Dry, Powder, Non-Standardized Extracts (Dry extract, Tincture, Fluid Extract, Decoction, Infusion)
0.5 - 9 grams dried root/rootlets, per day (Vuksan et al. 2008; Reay et al. 2006; Sievenpiper et al. 2006; Reay et al. 2005; Sunram-Lea et al. 2005; Kennedy et al. 2004; ESCOP 2003; Kennedy et al. 2002; Scholey and Kennedy 2002; Engels et al. 2001; Kennedy et al. 2001; Scaglione et al. 2001; Blumenthal et al. 2000; Engels et al. 1996; Scaglione et al. 1996; Gross et al. 1995; Scaglione et al. 1994; Scaglione et al. 1990; Petkov and Mosharrof 1987; D'Angelo et al. 1986; Soldati and Sticher 1980).
Traditional Chinese Medicine
Methods of preparation: Decoction, decoction concentrate
3 - 10 grams dried root, per day (PPRC 2015; Bensky et al. 2004, Chen and Chen 2004)
Methods of preparation: Powder (unextracted)
0.5 - 4 grams dried root, per day (PPRC 2015, Bensky et al. 2004, Chen and Chen 2004).
Direction(s) for use
No statement required.
Duration(s) of use
Ask a health care practitioner/health care provider/health care professional/doctor/physician for use beyond 3 months (Mills and Bone 2005; Blumenthal et al. 2000; Bradley 1992).
Risk Information
Caution(s) and warning(s)
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are pregnant or breastfeeding.
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you have diabetes (Brinker 2010; Vuksan et al. 2008; Seely et al. 2008; Sievenpiper et al. 2006; ESCOP 2003; Tetsutani et al. 2000; Sotaniemi et al. 1995; Chin 1991).
- Ask a health care practitioner/health care provider/health care professional/doctor/physician before use if you are taking antidepressant medication, blood thinners or digoxin (Brinker 2010; Lee et al. 2008a; Dasgupta and Reyes 2005; Janetzki and Morreale 1997; Gonzalez-Seijo et al. 1995; Shader and Greenblatt 1988; Jones and Runikis 1987; Shader and Greenblatt 1985).
Contraindication(s)
Traditional Chinese MedicineDo not use in cases of yin deficiency with heat signs, heat excess, or in the absence of significant qi deficiency (Bensky et al. 2004).
Known adverse reaction(s)
Stop use and ask a health care practitioner/health care provider/health care professional/doctor/physician if you experience insomnia, anxiety or headaches (Lee et al. 2008b; Vuksan et al 2008; de Andrade et al.2007; Sievenpiper et al. 2006; Coon and Ernst 2002; Ellis and Reddy 2002; Scaglione et al. 2001; Siegel 1979).
Non-medicinal ingredients
Must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in the database.
Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations.
Specifications
- The finished product specifications must be established in accordance with the requirements described in the Natural and Non-prescription Health Products Directorate (NNHPD) Quality of Natural Health Products Guide.
- The medicinal ingredient must comply with the requirements outlined in the NHPID.
EXAMPLE OF PRODUCT FACTS:
Consult the Guidance Document, Labelling of Natural Health Products for more details.
References Cited
- Bensky D, Clavey, Stöger E, Gamble A. Chinese Herbal Medicine: Materia Medica. 3rd edition. Seattle (WA): Eastland Press, Incorporated; 2004.
- BHP 1983: British Herbal Pharmacopoeia. Bournemouth (R-U): British Herbal Medical Association.
- Blumenthal M, Goldberg A, Brinkmann J, éditeurs. 2000. Herbal Medicine: Expanded Commission E Monographs. Boston (MA): Integrative Medicine Communications.
- Bone K. A clinical guide to blending liquid herbs: Herbal formulations for the individual patient. St. Louis (MI): Churchill Livingstone; 2003.
- Bradley PR, éditeur. 1992. British Herbal Compendium: A Handbook of Scientific Information on Widely Used Plant Drugs, Volume 1. Bournemouth (R-U): British Herbal Medicine Association.
- Brinker F. Herb Contraindications and Drug Interactions, 4e édition. Sandy (OR): Eclectic Medical Publications; 2010.
- Chen JK, Chen TT. Chinese Medical Herbology and Pharmacology. Crampton L, editor. City of Industry (CA): Art of Medicine Press Inc.; 2004.
- Chin RKH. 1991. Ginseng and common pregnancy disorders. Asia-Oceania Journal of Obstetrics and Gynaecology 17(4):379-380.
- Coon JT, Ernst E. 2002. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Safety 25(5):323-344.
- D'Angelo L, Grimaldi R, Caravaggi M, Marcoli M, Perucca E, Lecchini S, Frigo GM, Crema A. 1986. A double-blind, placebo-controlled clinical study on the effect of a standardized ginseng extract on psychomotor performance in healthy volunteers. Journal of Ethnopharmacology 16(1):15-22.
- Dasgupta A, Reyes MA. 2005. Effect of Brazillian, Indian, Siberian, Asian, and North American ginseng on serum digoxin measurement by immunoassays and binding of digoxin-like immunoreactive components of ginseng with Fab Fragment of antidigoxin antibody (Digiband). American Journal of Clinical Pathology 124(2):229-236.
- de Andrade E, de Masquita AA, de Almeida Claro J, de Andrade PM, Ortiz V, Paranhos M, Srougi M. 2007. Study of the efficacy of Korean Red Ginseng in the treatment of erectile dysfunction. Asian Journal of Andrology 9(2):241-244.
- Ellis JM, Reddy P. 2002. Effects of Panax ginseng on quality of life. The Annals of Pharmacotherapy 36(3):375-379.
- Engels HJ, Fahlman MM, Wirth JC. 2003. Effects of ginseng on secretory IgA, performance, and recovery from interval exercise. Medicine & Science in Sports & Exercise 35(4):690-696.
- Engels HJ, Kolokouri I, Cieslak TJ, Wirth JC. 2001. Effects of ginseng supplementation on supramaximal exercise performance and short-term recovery. Journal of Strength and Conditioning Research 15(3):290-295.
- Engels HJ, Said JM, Wirth JC. 1996. Failure of chronic ginseng supplementation to affect work performance and energy metabolism in healthy adult females. Nutrition Research 6(8):12951305.
- Engels HJ, Wirth JC. 1997. No ergogenic effects of ginseng (Panax ginseng C.A. Meyer) during graded maximal aerobic exercise. Journal of the American Dietetic Association 97(10):1110-1115.
- ESCOP 2003: European Scientific Cooperative on Phytotherapy Scientific Committee. 2003.ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products, 2e edition. Exeter (UK): European Scientific Cooperative on Phytotherapy and Thieme
- Gardner Z, McGuffin M, editors. American Herbal Products Association's Botanical Safety Handbook. Second Edition. Boca Raton (FL): Taylor and Francis Group; 2013.
- Gonzalez-Seijo JC, Ramos YM, Lastra I. 1995. Manic episode and ginseng: report of a possible case. Journal of Clinical Psychopharmacology 15(6):447-448.
- Gross D, Krieger D, Efrat R, Dayan. 1995. Ginseng extract G115 for the treatment of chronic respiratory diseases. Schweiz Zschr Ganzheits Medizin 1:29-33.
- Gross D, Shenkman Z, Bleiberg B, Dayan M, Gittelson M, Efrat R. 2002. Ginseng improves pulmonary functions and exercise capacity in patients with COPD. Monaldi Archives for Chest Disease 57(5-6):242-246.
- Heo JH, Lee ST, Chu K, Oh MJ, Park HJ, Shim JY, Kim M. 2008. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzeimer's disease. European Journal of Neurology 15(8):865-868.
- Hoffmann D. 2003. Medical Herbalism: The Science and Practice of Herbal Medicine. Rochester (VT): Healing Arts Press.
- Janetzki K, Morreale AP. 1997. Probable interaction between warfarin and ginseng. American Journal of Health-System Pharmacy 54(6):692-693.
- Jones BD, Runikis AM. 1987. Interaction of ginseng with phenelzine. Journal of Clinical Pharmacology 7(3):201-202.
- Kennedy DO, Haskell CF, Wesnes KA, Scholey AB. 2004. Improved cognitive performance in human volunteers following administration of guarana (Paullinia cupana) extract: comparison and interaction with Panax ginseng. Pharmacology, Biochemistry and Behavior 79(3):401-411.
- Kennedy DO, Scholey AB, Wesnes KA. 2002. Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng, and a ginkgo/ginseng combination to healthy young adults. Physiology & Behavior 75(5):739-751.
- Kennedy DO, Scholey AB, Wesnes KA. 2001. Dose dependent changes in cognitive performance and mood following acute administration of Ginseng to healthy young volunteers. Nutritional Neuroscience 4(4):295-310.
- Kim SH, Park KS, Chang MJ, Sung JH. 2005. Effects of panax ginseng extract on exercise-induced oxidative stress. Journal of Sports Medicine and Physical Fitness 45(2):178-182.
- Lee SH, Ahn YM, Ahn SY, Doo HK, Lee BC. 2008a. Interaction between warfarin and Panax ginseng in ischemic stroke patients. The Journal of Alternative and Complementary Medicine 14(6):715-721.
- Lee ST, Chu K, Sim JY, Heo JH, Kim M. 2008b. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Disease and Associated Disorders 22(3):222-226.
- Mills S, Bone K. 2005. The Essential Guide to Herbal Safety. St. Louis (MO): Elsevier Churchill Livingstone.
- Petkov VD, Mosharrof AH. 1987. Effects of standardized ginseng extract on learning, memory and physical capabilities. American Journal of Chinese Medicine 15(1-2):19-29.
- Ph. Eur. 2008: European Pharmacopoeia Commission. 2008. European Pharmacopoeia, 6th edition, Volume 1. Strasbourg (FR): Directorate for the Quality of Medicines and HealthCare of the Council of Europe (EDQM).
- PPRC 2015: Pharmacopoeia of the People's Republic of China, Volume I. English edition 2015. Beijing (CN): The State Pharmacopoeia Commission of the People's Republic of China.
- Reay JL, Kennedy DO, Scholey AB. 2006. Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained 'mentally demanding' tasks. Journal of Psychopharmacology 20(6):771-781.
- Reay JL, Kennedy DO, Scholey AB. 2005. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. Journal of Psychopharmacology 19(4):357-365.
- Scaglione F, Cattaneo G, Alessandria M, Cogo R. 1996. Efficacy and safety of the standardized ginseng extract G115 for potentiating vaccination against common cold and/or influenza syndrome. Drugs Under Experimental and Clinical Research 22(2):65-72.
- Scaglione F, Cogo R, Cocuzza C, Arcidiacono M, Beretta A. 1994. Immunomodulatory effects of Panax ginseng C.A. Meyer (G115) on alveolar macrophages from patients suffering with chronic bronchitis. International Journal of Immunotherapy 10(1):21-24.
- Scaglione F, Ferrara F, Dugnani S, Falchi M, Santoro G, Fraschini F. 1990. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Under Experimental and Clinical Research 16(10):537-542
- Scaglione F, Weiser K, Alessandria M. 2001. Effects of the standardised ginseng extract G115 in patients with chronic bronchitis. Clinical Drug Investigation 21(1):41-45.
- Schepdael PV. 1993. Les effets du ginseng G115 sur la capacité physique de sportifs d'endurance. Acta Therapeutica 19(4):337-347.
- Scholey AB, Kennedy DO. 2002. Acute, dose-dependent cognitive effects of Ginkgo biloba, Panax ginseng and their combination in healthy young volunteers: differential interactions with cognitive demand. Human Psychopharmacology 17(1):35-44.
- Seely D, Dugoua JJ, Perri D, Mills E, Koren G. 2008. Safety and efficacy of Panax ginseng during pregnancy and lactation. The Canadian Journal of Clinical Pharmacology 15(1):e87-e94.
- Shader RI, Greenblatt DJ. 1988. Bees, ginseng and MAOIs revisited. Journal of Clinical Psychopharmacology 8(4):235.
- Shader RI, Greenblatt DJ. 1985. Phenelzine and the dream machine – ramblings and reflections. Journal of Clinical Psychopharmacology 5(2):65.
- Siegel RK. 1979. Ginseng abuse syndrome. Problems with the panacea. The Journal of the American Medical Association 241(15):1614-1615.
- Sievenpiper JL, Sung MK, Buono MD, Seung-Lee K, Nam KY, Arnason JT, Leiter LA, Vuksan V. 2006. Korean red ginseng rootlets decrease acute postprandial glycemia: results from sequential preparation- and dose-finding studies. Journal of the American College of Nutrition 25(2):100-107.
- Soldati F, Sticher O. 1980. HPLC separation and quantitative determination of ginsenosides from Panax ginseng, Panax quinquefolium and from ginseng drug preparations. 2e communication. Planta Medica 39(4):348-357.
- Sorensen H, Sonne J. 1996. A double-masked study of the effects of ginseng on cognitive functions. Current Therapeutic Research 57(12):959-968.
- Sotaniemi EA, Haapakoski E, Rautio A. 1995. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care 18(10):1373-1375.
- Sünram-Lea SI, Birchall RJ, Wesnes KA, Petrini O. 2005. The effect of acute administration of 400 mg of Panax ginseng on cognitive performance and mood in healthy young volunteers. Current Topics in Nutraceutical Research 3(1):65-74.
- Tetsutani T, Yamamura M, Yamaguchi T, Onoyama O, Kono M. 2000. Can red ginseng control blood glucose in diabetic patients. The Ginseng Review 28:44-47.
- USDA 2024: United States Department of Agriculture, Agricultural Research Service (USDA ARS), Germplasm Resources Information Network (GRIN) - Global. U.S. National Plant Germplasm System. [Accessed 2024 January 11]. Available from: https://npgsweb.ars-rin.gov/gringlobal/taxon/taxonomysearch
- USP-NF 2023: United States Pharmacopeial Convention. United States Pharmacopeia and the National Formulary. Rockville (MD): The United States Pharmacopeial Convention.
- Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Buono MD, Lee KS, Leiter LA, Nam KY, Arnason JT, Choi M, Naeem A. 2008. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutrition, Metabolism & Cardiovascular Diseases 18(1):46-56.
- WHO 1999: World Health Organization (WHO) Monographs on Selected Medicinal Plants: Volume 1. Geneva (CHE): World Health Organization.
- Williamson EM. 2003. Potter’s Herbal Cyclopaedia: The Authoritative Reference work on Plants with a Known Medical Use. Saffron Walden (UK): The C.W. Daniel Company Limited.
- Winston D, Maimes S. Adaptogens: Herbs for strength, stamina and stress relief. Rochester (VT): Healing Arts Press; 2007.
- Wren RC. 1907. Potter’s Cyclopedia of Botanical Drugs and Preparations. London (UK): Potter and Clark.
References Reviewed
- Awang DVC, Fugh-Berman A. 2002. Herbal interactions with cardiovascular drugs. The Journal of Cardiovascular Nursing 16(4):64-70.
- Bahrke MS. 1997. Comments on ‘manic episode and ginseng: report of a possible case’. Journal of Clinical Psychopharmacology 17(2):140-141.
- Bahrke MS, Morgan WP. 1994. Evaluation of the ergogenic properties of ginseng. Sports Medicine 18(4):229-248.
- Bahrke MS, Morgan WP. 2000. Evaluation of the ergogenic properties of ginseng: an update. Sports Medicine 29(2):113-133.
- Bartram T. 1998. Bartram’s Encyclopedia of Herbal Medicine. Londres (R-U): Robinson Publishing Ltd.
- BHP 1996: British Herbal Pharmacopoeia. Bournemouth (R-U): The British Herbal Medicine Association.
- Blumenthal M. 2003. ABC Clinical Guide to Herbs. New York (NY): Theime.
- Bove M. 2001. An Encyclopedia of Natural Healing for Children and Infants, 2e edition. Toronto (ON): McGraw-Hill.
- BP 2008: British Pharmacopoeia Commission. 2007. British Pharmacopoeia 2008. Londres (RU): The Stationary Office.
- Bradley PR, éditeur. 2006. British Herbal Compendium: A Handbook of Scientific Information on Widely Used Plant Drugs, Volume 2. Bournemouth (R-U): British Herbal Medicine Association.
- Bucci LR. 2000. Selected herbs and human exercise performance. The American Journal of Clinical Nutrition 72(Suppl 2):624S-636S.
- Buettner C, Yeh GY, Phillips RS, Mittleman MA, Kaptchuk TJ. 2006. Systematic review of the effects of ginseng on cardiovascular risk factors. Complementary and Alternative Medicine 40(1):83-95.
- Cardinal BJ, Engels HJ. 2001. Ginseng does not enhance psychological well-being in healthy, young adults: results of a double-blind, placebo-controlled, randomized clinical trial. Journal of the American Dietetic Association 101(6):655-660.
- Chen K. 1981. The effect and abuse syndrome of ginseng. Journal of Traditional Chinese Medicine 1(1):69-72.
- Cho YK, Sung H, Lee HJ, Joo CH, Cho GJ. 2001. Long-term intake of Korean red ginseng in HIV-1-infected patients: development of resistance mutation to zidovudine is delayed. International Immunopharmacology 1(7):1295-1305.
- Choi HK, Choi YJ, Choi YD, Rha KH, Kim JH, Kim DK. 2002. SS-penogram: a new diagnostic test for erectile dysfunction. Yonsei Medical Journal 43(1):1-6.
- Choi HK, Seong DH, Rha KH. 1995. Clinical efficacy of Korean red ginseng for erectile dysfunction. International Journal of Impotence Research 7(3):181-186.
- Chosidow O, Dega H, Peytavin G. 1996. Ginseng as cause of Stevens-Johnson syndrome? The Lancet 348(9022):267.
- Coleman CI, Hebert JH, Reddy P. 2003. The effects of Panax ginseng on quality of life. Journal of Clinical Pharmacy and Therapeutics 28(1):5-15.
- Collomp K, Wright F, Collomp R, Shamari K, Bozzolan F, Préfaut C. 1996. Ginseng et exercice supramaximal. Science & Sports 11(4):250-251.
- Covington MB. 2001. Traditional Chinese medicine in the treatment of diabetes. Diabetes Spectrum 14(3):154-159.
- Dega H, Laporte JL, Francès C, Herson S, Chosidow O. 1996. Ginseng as a cause for StevensJohnson syndrome? The Lancet 347(9011):1344.
- Dukes MNG. 1978. Ginseng and mastalgia. British Medical Journal 1(6127):1621.
- Ellingwood F. 1983. American Materia Medica, Therapeutics and Pharmacognosy. Sandy (OR): Eclectic Medical Publications; [Réimpression de la publication originale de l919].
- Engelberg D, McCutcheon A. 2001. A case of ginseng-incduced mania. Journal of Clinical Psychopharmacology 21(5):535-537.
- Faleni R, Soldati F. 1996. Ginseng as cause of Stevens-Johnson syndrome? The Lancet 348(9022):267.
- Felter HW. 1983. The Eclectic Materia Medica, Pharmacology and Therapeutics. Sandy (OR):
- Eclectic Medical Publications; [Réimpression de la publication originale de 1922].
- Felter HW, Lloyd JU. 1983. King’s American Dispensatory, Volume 1, 18th edition. Sandy (OR): Eclectic Medical Publications; [Réimpression de la publication originale de 1898].
- Forgo I. 1982. The effect of different ginsenoside concentration on physical work capacity. Notabene Medici 12:721.
- Foro I. 1983. [Effect of drugs on physical and the hormonal system of athletes]. Wirkung von Pharmaka auf korperliche Leistung und Hormonsystem von Sportlern. 2. Mitteilung. Münchener Medizinische Wochenschrift 125(38):822-824. [en allemand].
- Forgo I. 1985. The duration of effect of the standardized ginseng extract G115 in healthy competitive athletes. Notabene Medici 15:636
- Forgo I, Kayasseh L, Staub JJ. 1981. [Effect of a standardized ginseng extract on general wellbeing, reaction time, lung function and gonadal hormones]. Einfluss eines standardisierten Ginseng-Extraktes auf das Allgemeinbefinden, die Reaktionsfahigkeit, Lungenfunktion und die gonadalen Hormone. Die Medizinische Welt 32(19):751-756. [en allemand].
- Forgo I, Kirchdorfer AM. 1981. [On the question of influencing the performance of top sportmen by means of biologically active substances]. Ginseng steigert die köperliche Leistung. Arztliche Praxis 33(44):1784-1786. [en allemand].
- Gaffney BT, Hügel HM, Rich PA. 2001. The effects of Eleutherococcus senticosus and Panax ginseng on steroidal hormone indices of stress and lymphocyte subset numbers in endurance athletes. Life Sciences 70(4):431-442.
- Gillis CN. 1997. Panax ginseng pharmacology: a nitric oxide link? Biochemical Pharmacology 54(1):1-8.
- Grieve M. 1971. A Modern Herbal, Volume 1. New York (NY): Dover Publications; [Réimpression de la publication Harcourt, Brace & Company de 1931].
- Gross D, Shenkman Z, Bleiberg B, Dayan M, Gittelson M, Efrat R. 2002. Ginseng improves pulmonary functions and exercise capacity in patients with COPD. Monaldi Archives for Chest Disease 57(5-6):242-246.
- Hallstrom C, Fulder S, Carruthers M. 1982. Effects of ginseng on the performance of nurses on night duty. Comparative Medicine East and West 6(4):277-282.
- Hong H, Ji YH, Hong JH, Nam KY, Ahn TY. 2002. A double-blind crossover study evaluating the efficacy of Korean red ginseng in patients with erectile dysfunction: a prelimary report. The Journal of Urology 168(5):2070-2073.
- Izzo AA, Di Carlo G, Borrelli F, Ernst E. 2005. Cardiovascular pharmacotherapy and herbal medicines: the risk of drug interaction. International Journal of Cardiology 98(1):1-14.
- Jang DJ, Lee MS, Shin BC, Lee YC, Ernst E. 2008. Red ginseng for treating erectile dysfunction: a systematic review. British Journal of Clinical Pharmacology 66(4):444-450.
- Jiang X, Blair EYL, McLachlan AJ. 2006. Investigation of the effects of herbal medicines on warfarin response in healthy subjects: a population pharmacokinetic-pharmacodynamix modeling approach. Journal of Clinical Pharmacology 46(11):1370-1378.
- Jiang X, Williams KM, LIauw WS, Ammit AJ, Roufogalis BD, Duke CC, Day RO, McLachlan AJ. 2004. Effect of St. John’s wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. British Journal of Clinical Pharmacology 57(5):592-599.
- Kaneko H, Nakanishi K. 2004. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. Journal of Pharmacological Sciences 95(2):158-162.
- Kaneko H, Nakanishi K, Kuwashima K, Ikeda K. 2000. Effects of the long-term administration of red ginseng on working stress and changes of physiological parameters of workers. A study at a care hospital for the aged (ROUJIN BYOUIN). Therapeutic Research 21(5):1451-1463. [résumé]
- Kang HY, Kim SH, Lee WJ, Byrne HK. 2002. Effects of ginseng ingestion on growth hormone, testosterone, cortisol, and insulin-like growth factor 1 responses to acute resistance exercise. Journal of Strength and Conditioning Research 16(2):179-183.
- Kennedy DO, Scholey AB. 2003. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacology, Biochemistry and Behavior 75(3):687-700.
- Kennedy DO, Scholey AB, Drewery L, Marsh VR, Moore B, Ashton H. 2003.
- Electroencephalograph effects of single doses of Ginkgo biloba and Panax ginseng in healthy young volunteers. Pharmacology, Biochemistry and Behavior 75(3):701-709.
- Kim JH, Park CY, Lee SJ. 2006. Effects of sun ginseng on subjective quality of life in cancer patients: a double-blind, placebo-controlled pilot trial. Journal of Clinical Pharmacy and Therapeutics 31(4):331-334.
- Klepser TB, Klepser ME. 1999. Unsafe and potentially safe herbal therapies. American Journal of Health-System Pharmacy 56(2):125-138.
- Kolokouri I, Engels HJ, Cieslack T, Wirth JC. 1999. Effect of chronic ginseng supplementation on short duration, supramaximal exercise test performance. Medicine & Science in Sports & Exercise 31(Suppl 5):S117.
- Kulaputana O, Thanakomsirichot S, Anomasiri W. 2007. Ginseng supplementation does not change lactate threshold and physical performances in physically active Thai men. Journal of the Medical Association of Thailand 90(6):1172-1179.
- Kwak HE, Kim SS, Kim YC, Jung SR, Kang HY, Lee CD. 2007. Effects of red ginseng intake on muscle injury due to eccentric exercise. Medicine & Science in Sports & Exercise 39(Suppl 5):S361.
- Lee HY, Kim CS. 1986. [Clinical investigation of Insam (Korean ginseng) on sexual potency]. Korean Journal of Urology 27(2):235-240. [en coréen]
- Lee FC, Ko JH, Park JK, Lee JS. 1987. Effects of Panax ginseng on blood alcohol clearance in man. Clinical and Experimental Pharmacology & Physiology 14(6):543-546.
- Lee HY, Paick JS, Lee SW. 1988. [Efficacy of ginseng extract on patients with oligospermia]. Korean Journal of Urology 29(8):950-960. [en coréen]
- Lust J. 1974. The Herb Book. New York (NY): Bantam Books Incorporated, published by arrangement with Benedict Lust Publications.
- Ma SW, Benzie IF, Chu TT, Fok BS, Tomlinson B, Critchley LA. 2008. Effect of Panax ginseng supplementation on biomarkers of glucose tolerance, antioxidant status and oxidative stress in type 2 diabetic subjects: results of a placebo-controlled human intervention trial. Diabetes, Obesity and Metabolism 10(11):1125-1127.
- Mills S, Bone K. 2000. Principles and Practice of Phytotherapy. Toronto (ON): Churchill Livingstone.
- Mills E, Duguoa J, Perri D, Koren G. 2006. Herbal Medicines in Pregnancy and Lactation. An Evidence-Based Approach. New York (NY): Taylor and Francis.
- Moerman DE. 1998. Native American Ethnobotany. Portland (OR): Timber Press.
- Murphy LL, Lee TJ. 2002. Ginseng, sex behavior, and nitric oxide. Annals of the New York Academy of Sciences 962:372-377.
- Nocerino E, Amato M, Izzo AA. 2000. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 71(Suppl 1):S1-S5.
- Palmer BV, Montgomery ACV, Monteiro JCMP. 1978. Gin seng and mastalgia. British Medical Journal 1(6122):1284.
- Persson J, Bringlov E, Nilsson LG, Nyberg L. 2004. The memory-enhancing effects of ginseng and ginkgo biloba in healthy volunteers. Psychopharmacology 172(4):430-434.
- Ravens JA, Edwards D. 2001. Roots: evolutionary origins and biogeochemical significance. Journal of Experimental Botany 52(90001):381-401.
- Reay JL, Kennedy DO, Scholey AB. 2006. The glycaemic effects of single doses of Panax ginseng in young healthy volunteers. British Journal of Nutrition 96(4):639-642.
- Reay JL, Scholey AB, Milne A, Fenwick J, Kennedy DO. 2008. Panax ginseng has no effect on indices of glucose regulation following acute or chronic ingestion in healthy volunteers. British Journal of Nutrition 19:1-6
- Ryu SJ, Chien YY. 1995. Ginseng-associated cerebral arteritis. Neurology 45:829-830.
- Salvati G, Genovesi G, Marcellini L, Paolini P, De Nuccio I, Pepe M, Re M. 1996. Effects of Panax ginseng C.A. Meyer saponins on male fertility. Panminerva Medica 38(4):249-254.
- SC 2007: Santé Canada. Base de données en ligne de Canada Vigilance : Le site web de recherche du PCSEIM. Ottawa (ON): Médicaments et produits de santé, Santé Canada. [Consulté le 23 avril 2009]. Disponible à : http://205.193.93.51/CADRMP/LocaleAction.do
- Schilcher, H. 1997. Phytotherapy in Paediatrics. Handbook for Physicians and Pharmacists. Stuttgart (D): Medpharm Scientific Publishers.
- Shou-zhong Y, translator. 2004. The Divine Farmer’s Materia Medica - A translation of the Shen Nong Ben Cao Jing. Boulder (CO): Blue Poppy Press.
- Sievenpiper JL, Arnason JT, Leiter LA, Vuksan V. 2003. Null and opposing effects of asian ginseng (Panax ginseng C.A Meyer) on acute glycemia: results of two acute dose escalation studies. Journal of the American College of Nutrition 22(6):524-532.
- Sievenpiper JL, Arnason JT, Leiter LA, Vuksan V. 2004. Decreasing, null and increasing effects of eight popular types of ginseng on acute postprandial glycemic indices in healthy humans: the role of ginsenosides. Journal of the American College of Nutrition 23(3):248-258.
- Soldati F. 1988. Immunological studies of ginseng. Proceedings of the 5th International Ginseng Symposium, Seoul: 108-114.
- Srisurapanon S, Rungroeng K, Apibal S, Cherdrugsi P, Siripol R, Vanich-Angkul V, Timvipark C. 1997. The effect of standardized ginseng extract on peripheral blood leukocytes and lymphocyte subsets: a preliminary study in young healthy adults. Journal of the Medical Association of Thailand 80(Suppl 1):S81-S85.
- Sticher O, Soldati F. HPLC Trennung and quantitative Bestimmung der Ginsenoside von Panax ginseng, Panax quinquefolium and von ginseng-spezialitaten. Planta Medica 36(1):30-42.
- Sung H, Kang SM, Lee MS, Kim TG, Cho YK. 2005. Korean red ginseng slows depletion of CD4 T cells in human immunodeficiency virus type 1-infected patients. Clinical and Diagnostic Laboratory Immunology 12(4):497-501.
- Teves M, Wright J, Welch M, Patton J, Mello R, Rock PB, Knapik JJ, Vogel JA, der
- Marderosian A. 1983. Effects of ginseng on repeated bouts of exhaustive exercise. Medicine & Science in Sports & Exercise 15(2):162.
- Tilgner S. 1999. Herbal Medicine from the Heart of the Earth. Creswell (OR): Wise Acre Press.
- Tode T, Kikuchi Y, Hirata J, Kita T, Nakata H, Nagata I. 1999. Effect of Korean red ginseng on psychological functions in patients with severe climacteric syndromes. International Journal of Gynecology & Obstetrics 67(3):169-174.
- von Ardenne M, Klemm W. 1987. Measurements of the increase in the difference between the arterial and venous Hb-O2 saturation obtained with daily administration of 200 mg standardized ginseng extract G115 for four weeks. Panminerva Medica 29:143-150.
- Vogel VJ. 1970. American Indian Medicine. Norman (OK): The University of Oklahoma Press.
- Vogler BK, Pittler MH, Ernst E. 1999. The efficacy of ginseng. A systematic review of randomized clinical trials. European Journal of Clinical Pharmacology 55(8):567-575.
- Weiss RF. 1998. Herbal Medicine. Gothenburg (SE), Beaconsfield (UK): AB Arcanum, Beaconsfield Publishers Ltd.
- Wiersema J, León B. 1999. World Economic Plants: A Standard Reference. Boca Raton (FL): CRC Press LLC.
- Williamson EM, Evans FJ, Wren RC. 1998. Potter's New Cyclopaedia of Botanical Drugs and Preparations. Saffron Walden (R-U): C.W. Daniel Company Limited.
- Wiseman N and Ye F. 1999. A Practical Dictionary of Chinese Medicine. 2e édition. Brookline (MA): Paradigm Publications.
- Yeh G, Eisenberg DM, Kaptchuk TJ, Phillips RS. 2003. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 26(4):1277-1294.
- Yeung H. 1998. Handbook of Chinese Herbal Formulas. Rosemeade (CA): Institute of Chinese Medicine.
- Ziemba AW, Chmura J, Kaciuba-Uscilko H, Nazar K, Wisnik P, Gawronski W. 1999. Ginseng treatment improves psychomotor performance at rest and during graded exercise in young athletes. International Journal of Sport Nutrition 9(4):371-377.