First Aid Antiseptics
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
FOREWORD
This monograph is intended to replace the existing First aid antiseptics monograph of February 9, 2016. This monograph describes the requirements necessary to receive marketing authorization [i.e. a Drug Identification Number (DIN) or Natural Product Number (NPN)], for topical minor wound cleansers. This monograph does not apply to antiseptic skin cleanser products for personal hand hygiene or to products for professional use. Products which do not meet the criteria outlined in this document can apply for market authorization outside of the monograph stream.
Applicants are reminded that first aid antiseptic skin cleansers, like other non-prescription drugs or natural health products, are subject to the Food and Drug Regulations or the Natural Health Products Regulations administered by the Natural and Non-prescription Health Products Directorate (NNHPD). This includes requirements related to labelling, manufacturing and product specifications. Additional information on labels, outside of those specified in the monograph, such as additional directions for use and/or non-therapeutic claims are acceptable as long as they meet the Guidelines for the Nonprescription and Cosmetic Industry Regarding Non-therapeutic Advertising and Labelling Claims, the Guidelines for Consumer Advertising of Health products for Nonprescription drugs, Natural Health Products, Vaccines and Medical Devices, and are not false, misleading or counterintuitive to the use of the product.
The development of this monograph is the result of a thorough review of existing regulations, guidance documents, policies and current practices within Health Canada and other leading regulatory agencies.
Note:The solidus (/) indicates that the terms and/or the statements are synonymous. Either term or statement may be selected by the applicant. Text in parentheses is additional optional information which can be included on the Product Licence Application form and label at the applicant's discretion.
Date
February 24, 2023
Medicinal Ingredient(s)
First aid antiseptics are classified as natural health products (NHPs) if they contain one ingredient from Table 1. Combinations are not permitted. Applicants applying for an NPN can access the appropriate forms and guidance at: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription.html.
First aid antiseptics are classified as non-prescription drugs if they contain one ingredient from Table 2. Combinations are not permitted. Applicants applying for a DIN can access the appropriate forms and guidance at: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents.html.
| Proper name(s) | Common name(s) | Source information | Quantity |
|---|---|---|---|
| Source ingredient(s) | |||
|
Hydrogen peroxide |
Hydrogen peroxide |
Hydrogen peroxide |
3% |
|
Povidone-iodine |
Povidone-iodine |
0.5 - 10% |
1At least one of the following references was consulted per proper name, common name and source information: USP 38; Gottschalck and McEwen 2006; O'Neil et al. 2001.
2At least one of the following references was consulted for the dosage: Khan and Naqvi 2006; Pray 2006; Carruthers-Czyzewski 1996; FDA 1991.
| Medicinal ingredient preferred name1 | Quantity1 |
|---|---|
|
Benzalkonium chloride |
0.1 - 0.13% |
|
Benzethonium chloride |
0.1 - 0.2% |
1FDA 1991.
Permitted Combinations of Ingredients:
No combinations are permitted.
Route of administration
Topical
Dosage form(s)
The following dosage forms are acceptable when used according to the requirements indicated in this monograph:
Acceptable dosage forms for Non-prescription drugs (NPDs):
Cream; Gel; Lotion; Ointment; Solution; Spray; Swab; Wipe.
Acceptable dosage forms for Natural Health Products (NHPs):
Cream (povidone iodine only); Gel (povidone iodine only); Lotion (povidone iodine only); Ointment (povidone iodine only); Solution; Spray; Swab, medicated; Topical liquid; Wipe, medicated.
Use(s) or Purpose(s)1
For all products:
- First aid antiseptic.
- For minor wound cleansing.
- Antiseptic/Medicated/Antibacterial wound cleanser.
- Kills (harmful) bacteria/germs.
- Effective in destroying (harmful) bacteria to provide antiseptic cleansing.
- Helps to prevent/reduce the risk of infection in minor cuts and scratches.
For products containing benzalkonium chloride or benzethonium chloride:
Helps to prevent/reduce the risk of infection in minor burns.
1Note: At least one of the following references was consulted: Pray 2006; Berardi et al. 2002; Carruthers-Czyzewski 1996; FDA 1991.
Dose(s)
Subpopulation(s)
Children 2 to 11 years, Adolescents 12 to 17 years, Adults 18 years and older.
Quantity(ies)
See Tables 1 and 2.
Direction(s) for use
For all products:
- Clean the affected area (FDA 1991).
- Apply a small amount to wound one to three times daily (FDA 1991).
- May be covered with a sterile bandage (FDA 1991).
- Supervise children when they use this product (FDA 1991).
For spray products:
Avoid inhaling or exposing others to spray.
Duration(s) of use
For occasional use.
Risk information
Caution(s) and Warning(s)
For all products:
- For external use only.
- When using this product avoid contact with eyes. If contact occurs, rinse thoroughly with water (FDA 1991).
- Stop use and ask/consult a doctor/physician/health care practitioner/health care provider/health care professional if symptoms worsen or persist after seven days, or if irritation develops (FDA 2013a, FDA 1991).
- Keep out of reach of children. If swallowed, call a poison control center or get medical help right away (FDA 2013a, Zimmerman 1993, FDA 1991).
For products containing povidone-iodine:
- Ask/consult a doctor/physician/health care practitioner/health care provider/health care professional prior to use on infants (FDA 2013a).
- Ask/consult a doctor/physician/health care practitioner/health care provider/health care professional prior to use if you have a thyroid disease (FDA 2013a).
For products containing benzalkonium chloride or benzethonium chloride:
Ask a doctor or pharmacist before use if you are pregnant or breastfeeding.
Contraindication(s)
For all products:
- Do not use in the eyes or over large areas of the body (FDA 1991).
- Do not use on deep or puncture wounds, animal bites or serious burns (FDA 1991).
For products containing povidone-iodine:
Do not use if you are pregnant or breastfeeding (FDA 2013a).
For products containing a medicinal ingredient from Table 2:
Do not use if you are allergic to any of the ingredients in the product.
Known Adverse Reaction(s)
For products containing povidone-iodine:
Rare anaphylactic reactions have been known to occur (Gray et al. 2013; Palobart et al. 2009; Yoshida et al. 2008).
Non-medicinal ingredients
Ingredients must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in that database, the Food and Drug Regulations, and the current Cosmetic Ingredient Hotlist, when relevant.
Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations or Food and Drug Regulations.
Specifications
For all products:
This monograph describes those requirements that are specific to this class of non-prescription drugs and to NHPs. Any change to the manufacturing process that impacts the safety and efficacy of the ingredients, such as the use of novel technology (e.g. nanotechnology), requires supporting data and will be reviewed outside the monograph.
For products containing Table 1 NHP medicinal ingredients only:
The finished product must be established in accordance with the requirements described in the NNHPD Quality of Natural Health Products Guide. The medicinal ingredient must comply with the requirements outlined in the NHPID.
For products containing Table 2 non-prescription drug medicinal ingredients:
Requirements described in the Regulations to the Food and Drugs Act must be met.
DRUG FACTS TABLE: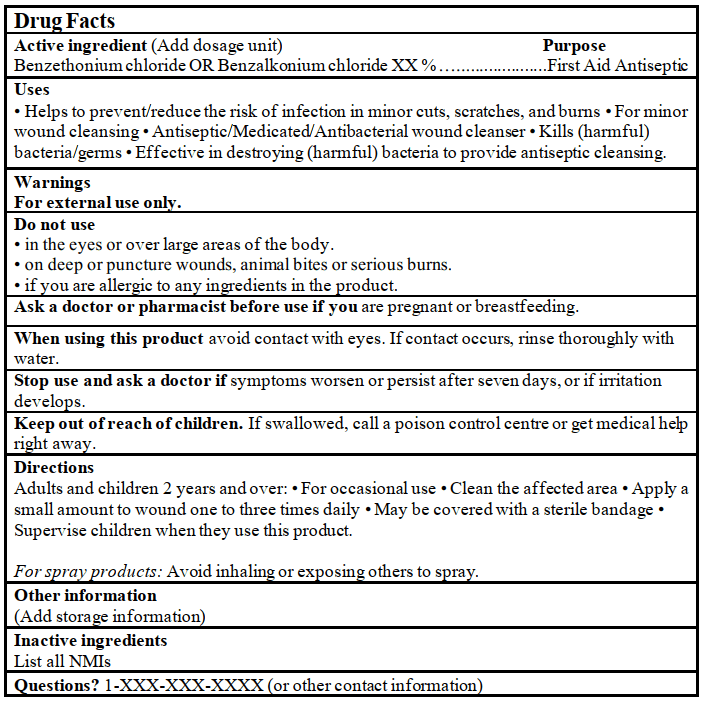
References cited
Berardi RR, DeSimone EM, Newton GD, Oszko MA, Popovich NG, Rollins CJ, Shimp LA, Tietze KJ, editors. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care, 13th edition. Washington (DC): American Pharmaceutical Association; 2002.
Carruthers-Czyzewski P, editor. Nonprescription Drug Reference for Health Professionals. 1st edition. Ottawa (ON): Canadian Pharmaceutical Association; 1996.
FDA 1991: United States Food and Drug Administration. 21 CFR Parts 333 and 369. Topical Antimicrobial Drug Products for Over-the-Counter Human Use; Tentative Final Monograph for First Aid Antiseptic Drug Products; [Internet]. Federal Register, Volume 56, Number 140, July 22, 1991. [Accessed 2014 April 10]. Available from: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Over-the-CounterOTCDrugs/StatusofOTCRulemakings/ucm079337.pdf
FDA 2013a: United States Food and Drug Administration. 21 CFR Parts 310 and 333. Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use; Proposed Amendment of the Tentative Final Monograph; Reopening of Administrative Record; [Internet]. Federal Register, Volume 78, Number 242, December 17, 2013. [Accessed 2014 April 10]. Available from: https://federalregister.gov/a/2013-29814
Gottschalck TE, McEwen GN, editors. International Cosmetic Ingredient Dictionary and Handbook. 11th edition. Washington (DC): Cosmetic, Toiletry and Fragrance Association; 2006.
Gray Pe, Katelaris CH, Lipson D. Recurrent anaphylaxis caused by topical povidone-iodine (Betadine). Journal of paediatrics and child health 2013;49(6):506-507.
Khan MN and AH Naqvi. Antiseptics, iodine, povidone iodine and traumatic wound cleansing. Journal of tissue viability 2006;16(4):6-10.
O'Neil MJ, Smith A, Heckelman PE, Budavari S, editors. Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th edition. Whitehouse Station (NJ): Merck & Co., Inc; 2001.
Palobart C, Cros J, Orsel I, Nathan N. Anaphylactic shock to iodinated povidone. Annales françaises d'anesthésie et de réanimation 2009;28(2):168-170.
Palobart C, Cros J, Orsel I, Nathan N. Anaphylactic shock to iodinated povidone. Annales françaises d'anesthésie et de réanimation 2009;28(2):168-170.
USP 38: The United States Pharmacopeia and the National Formulary (USP 38/NF 33). Rockville (MD): United States Pharmacopeial Convention, Inc.; 2015.
Yoshida K, Sakurai Y, Kawahara S, Takeda T, Ishikawa T, Murakami T, Yoshioka A. Anaphylaxis to polyvinylpyrrolidone in povidone-iodine for impetigo contagiosum in a boy with atopic dermatitis. International archives of allergy and immunology 2008;146:169-173.
Zimmerman DR. Zimmerman's Complete Guide to Nonprescription Drugs. Detroit (MI): Gale Research Inc.; 1993.
References reviewed
Arai K, Yamazaki M, Maeda T, Okura T, Tsuboi R. Influence of various treatments including povidone-iodine and healing stimulatory reagents in a rabbit ear wound model. International Wound Journal 2013;10(5):542-548.
Ascenzi JM. Handbook of Disinfectants and Antiseptics. New York (NY): Marcel Dekker; 1996.
Atiyeh BS, Dibo SA, Hayek SN. Wound cleansing, topical antiseptics and wound healing. International wound journal 2009;6(6):420-430.
AHFS 2014: American Hospital Formulary Service®. McEvoy GK (ed). AHFS Drug Information 2014®. [Internet] Published by Authority of the Board of the American Society of Health-System Pharmacists®, Bethesda, Maryland. [Accessed 2014 April 10]. Available from http://online.statref.com
Aiello AE, Larson EL, Levy SB. Consumer Antibacterial Soaps: Effective or Just Risky? Clinical Infectious Diseases. 2007;45(Supplement 2):S137-S147.
CDC 2005: Centers for Disease Control and Prevention. Guideline for Hand Hygiene in Health-Care Settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR 2002; Volume 51(No. RR-16).
FDA 1994: United States Food and Drug Administration. 21 CFR Parts 333 and 369. Topical Antimicrobial Drug Products for Over-the-Counter Human Use; Tentative Final Monograph for Health-Care Antiseptic Drug Products; [Internet]. Federal Register, Volume 59, Number 116, June 17, 1994. [Accessed 2014 April 10]. Available from: http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4098B1_02_03-FDA-TAB1.pdf
FDA 2013b: United States Food and Drug Administration. FDA Drug Safety Communication: FDA requests label changes and single-use packaging for some over-the-counter topical antiseptic products to decrease risk of infection; [Internet]. Drug Safety Communication, November 13, 2013. [Accessed 2014 April 10]. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM374870.pdf
Geronemus RG, Mertz PM, Eaglstein WH. Wound healing. The effects of topical antimicrobial agents. Archives of dermatology 1979;115(11):1311-1314.
Gilmore OJ, Reid C, Strokon A. A study of the effect of povidone-iodine on wound healing. Postgraduate medical journal 1977;53(617):122-125.
Goldenheim PD. An appraisal of povidone-iodine and wound healing. Postgraduate medical journal 1993;69(Suppl 3):S97-S105.
Gravett A, Sterner S, Clinton JE, Ruiz E. A trial of povidone-iodine in the prevention of infection in sutured lacerations. Annals of emergency medicine 1987;16(2):167-171.
Guideline for Hand Hygiene in Health-Care Settings. Morbidity and Mortality Weekly Report. Volume 51, Number RR-16, October 25, 2002.
Health Canada 2003: Guidance for Industry: Product Monograph. Health Canada, October 2003.
Health Canada 2005: Draft Guidance for Industry: Impurities in Existing Drug Substances and Products. Health Canada, September 2005.
Health Canada 2009: Guidance Document: Human-Use Antiseptic Drugs. Health Canada, December 2009. [Accessed 2015 September 18]. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/antiseptic_guide_ld-eng.php#star91
Juhasz I. Experiences with the use of povidone-iodine-containing local therapeutics in dermatological surgery and in the treatment of burns: testing for allergic sensitization in postsurgery patients. Dermatology 2002;204(Suppl 1):52-58.
Kramer SA. Effect of povidone-iodine on wound healing: a review. Journal of vascular nursing 1999;17(1):17-23.
Nili F, Hantoushzadeh S, Alimohamadi A, Shariat M, Rezaeizadeh G. Iodine-containing disinfectants in preparation for caesarean section: impact on thyroid profile in cord blood. Postgraduate medical journal 2015 -133540. doi: 10.1136. Epub ahead of print.
Norman D. The use of povidone-iodine in superficial partial-thickness burns. British journal of nursing 2003;12(6 Suppl):S30-S36.
Peter FW, Li-Peuser H, Vogt PM, Muehlberger T, Homann HH, Steinau HU. The effect of wound ointments on tissue microcirculation and leucocyte behaviour. Clinical and experimental dermatology 2002;7(1):51-55.
Roberts AH, Roberts FE, Hall RI, Thomas IH. A prospective trial of prophylactic povidone iodine in lacerations of the hand. Journal of hand surgery 1985;10(3):370-374.
Sweetman SC, editor. Martindale: The Complete Drug Reference. 33rd edition. London (GB): Pharmaceutical Press; 2002.
Trampuz AT and Widmer AF. Hand hygiene: a frequently missed lifesaving opportunity during patient care. Mayo Clinic proceedings 2004;79:109-116.
WHO 2005: WHO guidelines on hand hygiene in health care. Geneva (Switzerland): World Health Organization Press; 2005.
Zahidi A, Draoui M, Mestassi M. Iodine status and the use of iodized antiseptics in the mother-newborn pair. Therapie 1999;54(5)545-548.
Zhen ZJ, Lai ECH, Lee QH, Chen HW, Lau WY, Wang FJ. Conventional wound management versus a closed suction irrigation method for infected laparotomy wound - A comparative study. International Journal of Surgery 2011;9:378-381.