BILBERRY - VACCINIUM MYRTILLUS - Oral
Help on accessing alternative formats, such as Portable Document Format (PDF), Microsoft Word and PowerPoint (PPT) files, can be obtained in the alternate format help section.
This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization. It is not intended to be a comprehensive review of the medicinal ingredient.
Notes
- Text in parentheses is additional optional information which can be included on the PLA and product label at the applicant's discretion.
- The solidus (/) indicates that the terms and/or statements are synonymous. Either term or statement may be selected by the applicant.
Date
Febuary 23, 2024
Proper name(s), Common name(s), Source information
| Proper name(s) | Common name(s) | Source information | ||
|---|---|---|---|---|
| Source material(s) | Part(s) | Preparation(s) | ||
| Vaccinium myrtillus |
|
Vaccinium myrtillus | Fruit | Dry |
References: Proper name: USDA 2023; Common names: Gardner and McGuffin 2013; Source information: Blumenthal et al. 2000.
Route of Administration
Oral (Blumenthal et al. 2000)
Dosage Form(s)
This monograph excludes foods or food-like dosage forms as indicated in the Compendium of Monographs Guidance Document.
Acceptable dosage forms for oral use are indicated in the dosage form drop-down list of the web-based Product Licence Application form for Compendial applications.
Use(s) or Purpose(s)
- (Traditionally) used in Herbal Medicine as an astringent to help relieve diarrhoea (ESCOP 2003; Blumenthal et al. 2000; Mills and Bone 2000; Grieve 1971; Felter and Lloyd 1983).
- Source of antioxidants/Provides antioxidants (Upton 2001; Mills and Bone 2000)
- Source of antioxidants/Provides antioxidants that help fight/protect (cell) against/reduce (the oxidative effect of/the oxidative damage caused by/cell damage caused by) free radicals (Upton 2001; Mills and Bone 2000).
- Used in Herbal Medicine to help slow the progression of disorders of the eye, such as diabetic and hypertensive retinopathy, and macular degeneration (Mills and Bone 2005; Blumenthal 2003; Upton 2001; Morazzoni and Bombardelli 1996).
- Used in Herbal Medicine to help relieve symptoms related to non-complicated chronic venous insufficiency (CVI), such as sensation of swelling, heaviness and tingling of the legs (Barnes 2007; Mills and Bone 2005; ESCOP 2003; Upton 2001).
Notes
- The above uses can be combined on the product label if from the same traditional or non-traditional system of medicine (e.g. Used in Herbal Medicine to help slow the progression of disorders of the eye, such as diabetic and hypertensive retinopathy, and macular degeneration and to help relieve symptoms related to non-complicated chronic venous insufficiency (CVI), such as sensation of swelling, heaviness and tingling of the legs).
- For multi-ingredient products:
- To prevent the product from being represented as a "traditional medicine", any indicated traditional use claim must refer to the specific medicinal ingredient(s) and recognized traditional system of medicine from which the claim originates when 1) both traditional and modern claims are present or 2) when claims originate from multiple systems of traditional medicine (e.g. Bilberry is traditionally used in Herbal Medicine as an astringent to help relieve diarrhoea).
- When ALL of the medicinal ingredients (MIs) in the product are used within the SAME identified system of traditional medicine AND the product makes ONLY traditional claims, listing of MIs in the traditional claim(s) is not required
Dose(s)
Subpopulation(s)
Adults 18 years and older
Quantity(ies)
Slowing the progression of disorders of the eye and relieving symptoms related to non-complicated CVI
Methods of preparation: Standardized extracts (Dry extract)
160 - 480 milligrams of dried extract standardized to 36% anthocyanins, per day; Not to exceed 160 milligrams per single dose (USP-NF 2023; Blumenthal 2003; ESCOP 2003; Upton 2001).
All other uses
Methods of preparation: Dry, Dry standardized, Powder, Powder standardized, Standardized and Non-Standardised Extracts (Dry extract, Tincture, Fluid extract, Decoction, Infusion)
1.8 - 75 grams of dried fruit, per day (USP-NF 2023; Barnes 2007; ESCOP 2003; Blumenthal et al. 2000; Grieve 1971).
Standardized preparations only: not to exceed 36% anthocyanins
Direction(s) for use
No statement required.
Duration(s) of Use
No statement required.
Risk Information
Caution(s) and warning(s)
Relief of diarrhoea
Ask a health care practitioner/health care provider/health care professional/doctor/physician if symptoms persist or worsen.
Slowing the progression of disorders of the eye and to relieve symptoms related to non-complicated CVI
Ask a health care practitioner/health care provider/health care professional/doctor/physician if symptoms worsen.
Contraindication(s)
No statement required.
Known adverse reaction(s)
No statement required.
Non-medicinal ingredients
Must be chosen from the current Natural Health Products Ingredients Database (NHPID) and must meet the limitations outlined in the database.
Storage conditions
Must be established in accordance with the requirements described in the Natural Health Products Regulations.
Specifications
- The finished product specifications must be established in accordance with the requirements described in the Natural and Non-prescription Health Products Directorate (NNHPD) Quality of Natural Health Products Guide.
- The medicinal ingredient must comply with the requirements outlined in the NHPID.
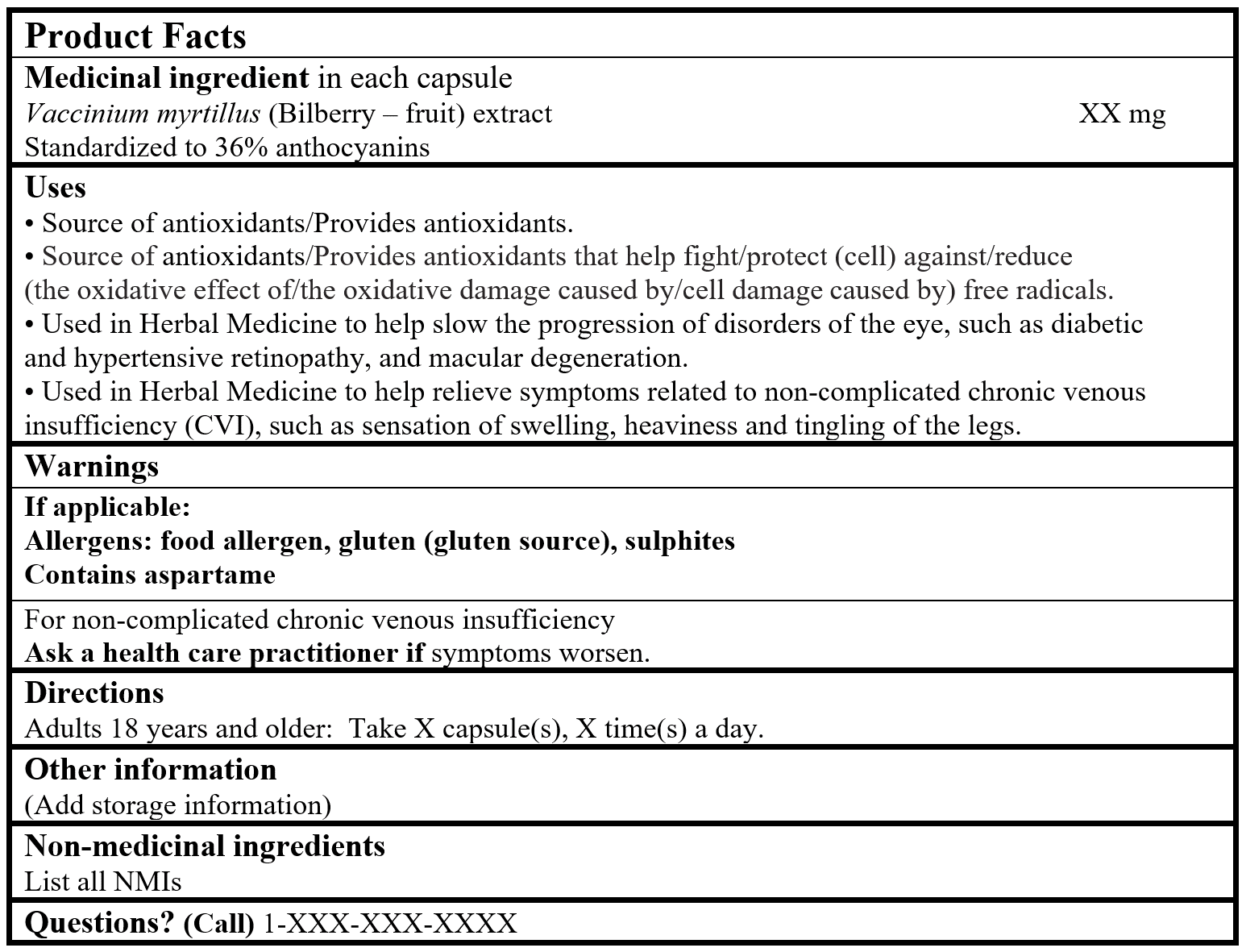
EXAMPLE OF PRODUCT FACTS:
Consult the Guidance Document, Labelling of Natural Health Products for more details.
References Cited
- Barnes J, Anderson LA, Philipson JD. 2007. Herbal Medicines, 3rd edition. London (GB): The Pharmaceutical Press.
- Blumenthal M. 2003. ABC Clinical Guide to Herbs. New York (NY): Thieme.
- Blumenthal M, Goldberg A, Brinkmann J, editors. 2000. Herbal Medicine: Expanded Commission E Monographs. Boston (MA): Integrative Medicine Communications.
- ESCOP 2003: European Scientific Cooperative on Phytotherapy Scientific Committee. 2003.ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products, 2nd edition. Exeter (GB): European Scientific Cooperative on Phytotherapy and Thieme.
- Felter HW, Lloyd JU. 1983. King's American Dispensatory, Volume 2, 18th edition. Sandy (OR): Eclectic Medical Publications [Reprint of 1898 original].
- Gardner Z, McGuffin M. editors. 2013. American Herbal Products Association's Botanical Safety Handbook, 2nd edition. Boca Raton (FL): CRC Press.
- Grieve M. 1971. A Modern Herbal, Volume 1. New York (NY): Dover Publications [Reprint of 1931 Harcourt, Brace & Company publication].
- Mills S, Bone K. 2005. The Essential Guide to Herbal Safety. St. Louis (MO): Churchill Livingstone.
- Mills S, Bone K. 2000. Principles and Practice of Phytotherapy. Toronto (ON): Churchill Livingstone.
- Morazzoni P, Bombardelli E. 1996. Vaccinium myrtillus L. Fitoterapia 67(1):3-29.
- Upton R, editor. 2001. American Herbal Pharmacopoeia and Therapeutic Compendium: Bilberry Fruit Vaccinium mysrtillus L.: Standards of Analysis, Quality Control and Therapeutics. Santa Cruz (CA): American Herbal Pharmacopoeia.
- USDA 2023: United States Department of Agriculture, Agricultural Research Service, National Genetic Resources Program. Germplasm Resources Information Network (GRIN). – Global. U.S. National Plant Germplasm System. [Accessed 2023 December 27]. Available from: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysearch
- USP-NF 2023: United States Pharmacopeia and the National Formulary. Rockville (MD): United States Pharmacopeial Convention, Inc.; 2023.
References Reviewed
- Brinker F. 2001. Herb Contraindications and Drug Interactions, 3rd edition. Sandy (OR): Eclectic Medical Publications.
- Canter PH, Ernst E. 2004. Anthocyanosides of Vaccinium myritillus (bilberry) for night vision - a systematic review of placebo-controlled trials. Survey of Opthalmology 49(1):38-50.
- Jang YP, Zhou J, Nakanishi K, Sparrow JR. 2005. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochemistry and Photobiology 81(3):529-536.
- Lee J, Lee HK, Kim CY, Hong YJ, Choe CM, You TW, Seong GJ. 2005. Purified high-dose anthocyanoside oligomer administration improves nocturnal vision and clinical symptoms in myopia subjects. British Journal of Nutrition 93:895-899.
- Levy Y, Glovinski Y. 1998. The effect of anthocyanosides on night vision. Eye 12:967-969.
- McGuffin M, Hobbs C, Upton R, Goldberg A, editors. 1997. American Herbal Products Association's Botanical Safety Handbook. Boca Raton (FL): CRC Press.
- Muth ER, Laurent JM, Jasper P. 2000. The effect of bilberry nutritional supplementation on night vision acuity and contrast sensitivity. Alternative Medicine Review 5(2):164-173.
- Sparrow JR, Vollmer-Snarr HR, Zhou J, Jang PY, Jockusch. 2003. A2E-epoxides damage DNA in retinal pigment epithelial cells. Journal of Biological Chemistry 278(20):18207-18213.
- Steigwalt RD Jr, Gianni B, Paolo M, Bombardelli E, Burki C, Schönlau F. 2008. Effects of Mirtogenol® on ocular blood flow and intraocular hypertension in asymptomatic subjects. Molecular Vision 14:1288-1292.
- Zadok D, Levy Y, Glovinski Y. 1999. The effect of anthocyanosides in multiple oral dose on night vision. Eye 13:734-736.